Comparison of human papillomavirus-based cervical cancer screening strategies in Tanzania among women with and without HIV
- PMID: 36093587
- PMCID: PMC10087897
- DOI: 10.1002/ijc.34283
Comparison of human papillomavirus-based cervical cancer screening strategies in Tanzania among women with and without HIV
Abstract
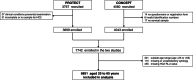
Cervical cancer is the most common female cancer in Eastern Africa, and the World Health Organization (WHO) recommends human papillomavirus (HPV)-based screening as a key element to eliminate the disease. In this cross-sectional study from Tanzania, we compared nine HPV-based cervical cancer screening strategies, including HPV testing at standard cut-off; HPV testing at increased viral load cut-offs; HPV testing with partial/extended genotyping, and HPV testing with visual inspection with acetic acid (VIA). We pooled data collected during 2008 to 2009 and 2015 to 2017 from 6851 women aged 25 to 65. Cervical cytology samples were HPV tested with Hybrid Capture 2, and HPV positive samples were genotyped with INNO-LiPA Extra II. Human immunodeficiency virus (HIV) testing and VIA were done according to local standards. We calculated sensitivity, specificity, positive and negative predictive value of screening strategies, with high-grade cytological lesions as reference, separately for women with and without HIV. HPV testing at standard cut-off (1.0 relative light units [RLU]) had highest sensitivity (HIV+: 97.8%; HIV-: 91.5%), but moderate specificity (HIV+: 68.1%; HIV-: 85.7%). Increasing the cut-off for HPV positivity to higher viral loads (5.0/10.0 RLU) increased specificity (HIV+: 74.2%-76.5%; HIV-: 89.5%-91.2%), with modest sensitivity reductions (HIV+: 91.3%-95.7%; HIV-: 83.5%-87.8%). Limiting test positivity to HPV types 16/18/31/33/35/45/52/58 improved specificity while maintaining high sensitivity (HIV+: 90.2%; HIV-: 81.1%). Triage with VIA and/or partial genotyping for HPV16/18 or HPV16/18/45 had low sensitivities (≤65%). In conclusion, HPV testing alone, or HPV testing with extended genotyping or increased viral load cut-offs, may improve cervical cancer screening in Sub-Saharan Africa.
Keywords: Africa; cervical cancer; human papillomavirus; prevention; screening.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Crispin Kahesa, Louise T. Thomsen, Ditte S. Linde, Bariki Mchome, Johnson Katanga, Patricia Swai, Rachel Manongi, Myassa Kjaerem, Marianne Waldstrøm, Julius Mwaiselage and Vibeke Rasch report no potential conflicts of interest. Susanne K. Kjær reports that she previously received research grants from Merck through the affiliating institution. Thomas Iftner reports that his hosting institution received research grants from Hologic Inc and Becton Dickinson.
Figures

References
-
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Fact Sheet, Cervix Uteri. Lyon, France: International Agency for Research on Cancer; 2020.
-
- World Health Organization . WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. 2nd ed. Geneva: WHO; 2021. - PubMed
-
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5‐14. - PubMed

