Arbuscular mycorrhizal fungi influence host infection during epidemics in a wild plant pathosystem
- PMID: 36093733
- PMCID: PMC9827988
- DOI: 10.1111/nph.18481
Arbuscular mycorrhizal fungi influence host infection during epidemics in a wild plant pathosystem
Abstract
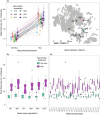
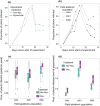
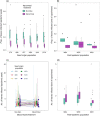
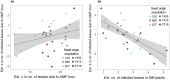
While pathogenic and mutualistic microbes are ubiquitous across ecosystems and often co-occur within hosts, how they interact to determine patterns of disease in genetically diverse wild populations is unknown. To test whether microbial mutualists provide protection against pathogens, and whether this varies among host genotypes, we conducted a field experiment in three naturally occurring epidemics of a fungal pathogen, Podosphaera plantaginis, infecting a host plant, Plantago lanceolata, in the Åland Islands, Finland. In each population, we collected epidemiological data on experimental plants from six allopatric populations that had been inoculated with a mixture of mutualistic arbuscular mycorrhizal fungi or a nonmycorrhizal control. Inoculation with arbuscular mycorrhizal fungi increased growth in plants from every population, but also increased host infection rate. Mycorrhizal effects on disease severity varied among host genotypes and strengthened over time during the epidemic. Host genotypes that were more susceptible to the pathogen received stronger protective effects from inoculation. Our results show that arbuscular mycorrhizal fungi introduce both benefits and risks to host plants, and shift patterns of infection in host populations under pathogen attack. Understanding how mutualists alter host susceptibility to disease will be important for predicting infection outcomes in ecological communities and in agriculture.
Keywords: Plantago lanceolata; Podosphaera plantaginis; mutualism; mycorrhizal fungi; plant disease; plant pathogen; powdery mildew; protective symbiont.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Figures
References
-
- Azcón‐Aguilar C, Barea JM. 1996. Arbuscular mycorrhizas and biological control of soil‐borne plant pathogens – an overview of the mechanisms involved. Mycorrhiza 6: 457–464.
-
- Bachelot B, Uriarte M, McGuire K. 2015. Interactions among mutualism, competition, and predation foster species coexistence in diverse communities. Theoretical Ecology 8: 297–312.
-
- Barea JM, Azcón R, Azcón‐Aguilar C. 2002. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81: 343–351. - PubMed
-
- Bever JD, Mangan SA, Alexander HM. 2015. Maintenance of plant species diversity by pathogens. Annual Review of Ecology, Evolution, and Systematics 46: 305–325.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

