Phase 1 Evaluation of Elezanumab (Anti-Repulsive Guidance Molecule A Monoclonal Antibody) in Healthy and Multiple Sclerosis Participants
- PMID: 36093738
- PMCID: PMC10100020
- DOI: 10.1002/ana.26503
Phase 1 Evaluation of Elezanumab (Anti-Repulsive Guidance Molecule A Monoclonal Antibody) in Healthy and Multiple Sclerosis Participants
Abstract
Objective: This study was undertaken to describe the safety, tolerability, pharmacokinetics, and immunogenicity of elezanumab (ABT-555), a fully human monoclonal antibody (mAb) directed against repulsive guidance molecule A (RGMa), in healthy and multiple sclerosis (MS) study participants.
Methods: The single-center, first-in-human, single ascending dose (SAD) study evaluated elezanumab (50-1,600mg intravenous [IV] and 150mg subcutaneous) in 47 healthy men and women. The multicenter multiple ascending dose (MAD; NCT02601885) study evaluated elezanumab (150mg, 600mg, and 1,800mg) in 20 adult men and women with MS, receiving either maintenance or no immunomodulatory treatment.
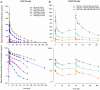
Results: No pattern of study drug-related adverse events was identified for either the SAD or MAD elezanumab regimens. Across both studies, the Tmax occurred within 4 hours of elezanumab IV infusion, and the harmonic mean of t1/2 ranged between 18.6 and 67.7 days. Following multiple dosing, elezanumab Cmax , area under the curve, and Ctrough increased dose-proportionally and resulted in dose-dependent increases in elezanumab cerebrospinal fluid (CSF) concentrations. Elezanumab CSF penetration was 0.1% to 0.4% across both studies, with CSF levels of free RGMa decreased by >40%. Changes in CSF interleukin-10 (IL-10) and free RGMa demonstrated dose/exposure-dependence.
Interpretation: The elezanumab pharmacokinetic profile supports monthly, or bimonthly, administration of up to 1,800mg with the option of a loading dose of 3,600mg. Elezanumab partitioning into CSF is within the range expected for mAbs. Reduced CSF levels of free RGMa demonstrate central nervous system target binding of elezanumab with an apparent maximal effect at 1,800mg IV. Exposure-associated increases in CSF IL-10, an anti-inflammatory cytokine with neuroprotective/neurorestorative properties, support potential pathway modulation in MS participants. ANN NEUROL 2023;93:285-296.
© 2022 AbbVie Inc and The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
H.V.K., M.R.R., A.Z., and W.L. are AbbVie employees and may hold stock or options. T.P.M. was an AbbVie employee at the time this work was conducted and may hold stock or options. B.A.C.C. has received personal compensation for consulting from Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Gossamer Bio, Horizon, Neuron23, Novartis, Sanofi, TG Therapeutics, and Therini and has received research support from Genentech.
Figures



References
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. - PubMed
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 2008;31:247–269. - PubMed
-
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron 2006;52:61–76. - PubMed
-
- De Stefano N, Narayanan S, Matthews PM, et al. In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain 1999;122:1933–1939. - PubMed
-
- Ferguson B, Matyszak MK, Esiri MM, et al. Axonal damage in acute multiple sclerosis lesions. Brain 1997;120:393–399. - PubMed

