Local neuronal excitation and global inhibition during epileptic fast ripples in humans
- PMID: 36093747
- PMCID: PMC9924905
- DOI: 10.1093/brain/awac319
Local neuronal excitation and global inhibition during epileptic fast ripples in humans
Abstract
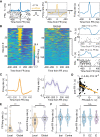
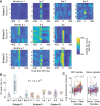
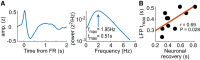
Understanding the neuronal basis of epileptic activity is a major challenge in neurology. Cellular integration into larger scale networks is all the more challenging. In the local field potential, interictal epileptic discharges can be associated with fast ripples (200-600 Hz), which are a promising marker of the epileptogenic zone. Yet, how neuronal populations in the epileptogenic zone and in healthy tissue are affected by fast ripples remain unclear. Here, we used a novel 'hybrid' macro-micro depth electrode in nine drug-resistant epileptic patients, combining classic depth recording of local field potentials (macro-contacts) and two or three tetrodes (four micro-wires bundled together) enabling up to 15 neurons in local circuits to be simultaneously recorded. We characterized neuronal responses (190 single units) with the timing of fast ripples (2233 fast ripples) on the same hybrid and other electrodes that target other brain regions. Micro-wire recordings reveal signals that are not visible on macro-contacts. While fast ripples detected on the closest macro-contact to the tetrodes were always associated with fast ripples on the tetrodes, 82% of fast ripples detected on tetrodes were associated with detectable fast ripples on the nearest macro-contact. Moreover, neuronal recordings were taken in and outside the epileptogenic zone of implanted epileptic subjects and they revealed an interlay of excitation and inhibition across anatomical scales. While fast ripples were associated with increased neuronal activity in very local circuits only, they were followed by inhibition in large-scale networks (beyond the epileptogenic zone, even in healthy cortex). Neuronal responses to fast ripples were homogeneous in local networks but differed across brain areas. Similarly, post-fast ripple inhibition varied across recording locations and subjects and was shorter than typical inter-fast ripple intervals, suggesting that this inhibition is a fundamental refractory process for the networks. These findings demonstrate that fast ripples engage local and global networks, including healthy tissue, and point to network features that pave the way for new diagnostic and therapeutic strategies. They also reveal how even localized pathological brain dynamics can affect a broad range of cognitive functions.
Keywords: epilepsy; high frequency oscillations; micro-electrodes; single unit; tetrodes.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Binnie CD. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–730. - PubMed
-
- Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. - PubMed
-
- Lambert I, Tramoni-Negre E, Lagarde S, et al. . Hippocampal interictal spikes during sleep impact long-term memory consolidation. Ann Neurol. 2020;87:976–987. - PubMed

