Distinct roles of the Chlamydia trachomatis effectors TarP and TmeA in the regulation of formin and Arp2/3 during entry
- PMID: 36093837
- PMCID: PMC9659389
- DOI: 10.1242/jcs.260185
Distinct roles of the Chlamydia trachomatis effectors TarP and TmeA in the regulation of formin and Arp2/3 during entry
Abstract
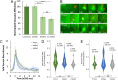
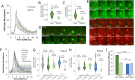
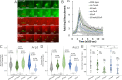
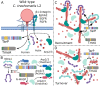
The obligate intracellular pathogen Chlamydia trachomatis manipulates the host actin cytoskeleton to assemble actin-rich structures that drive pathogen entry. The recent discovery of TmeA, which, like TarP, is an invasion-associated type III effector implicated in actin remodeling, raised questions regarding the nature of their functional interaction. Quantitative live-cell imaging of actin remodeling at invasion sites revealed differences in recruitment and turnover kinetics associated with the TarP and TmeA pathways, with the former accounting for most of the robust actin dynamics at invasion sites. TarP-mediated recruitment of actin nucleators, i.e. formins and the Arp2/3 complex, was crucial for rapid actin kinetics, generating a collaborative positive feedback loop that enhanced their respective actin-nucleating activities within invasion sites. In contrast, the formin Fmn1 was not recruited to invasion sites and did not collaborate with Arp2/3 within the context of TmeA-associated actin recruitment. Although the TarP-Fmn1-Arp2/3 signaling axis is responsible for the majority of actin dynamics, its inhibition had similar effects as the deletion of TmeA on invasion efficiency, consistent with the proposed model that TarP and TmeA act on different stages of the same invasion pathway.
Keywords: Chlamydia invasion; Actin kinetics; Formin; TarP; TmeA.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Dynamin-dependent entry of Chlamydia trachomatis is sequentially regulated by the effectors TarP and TmeA.Nat Commun. 2024 Jun 10;15(1):4926. doi: 10.1038/s41467-024-49350-6. Nat Commun. 2024. PMID: 38858371 Free PMC article.
-
Chlamydia trachomatis TmeA Directly Activates N-WASP To Promote Actin Polymerization and Functions Synergistically with TarP during Invasion.mBio. 2021 Jan 19;12(1):e02861-20. doi: 10.1128/mBio.02861-20. mBio. 2021. PMID: 33468693 Free PMC article.
-
The Chlamydia trachomatis secreted effector TmeA hijacks the N-WASP-ARP2/3 actin remodeling axis to facilitate cellular invasion.PLoS Pathog. 2020 Sep 18;16(9):e1008878. doi: 10.1371/journal.ppat.1008878. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32946535 Free PMC article.
-
Chlamydia trachomatis invasion: a duet of effectors.Biochem Soc Trans. 2025 Mar 24;0(0):363-70. doi: 10.1042/BST20240800. Biochem Soc Trans. 2025. PMID: 40131835 Free PMC article. Review.
-
Bacterial subversion of host cytoskeletal machinery: hijacking formins and the Arp2/3 complex.Bioessays. 2014 Jul;36(7):687-96. doi: 10.1002/bies.201400038. Epub 2014 May 21. Bioessays. 2014. PMID: 24849003 Review.
Cited by
-
Dynamin-dependent entry of Chlamydia trachomatis is sequentially regulated by the effectors TarP and TmeA.Res Sq [Preprint]. 2023 Sep 27:rs.3.rs-3376558. doi: 10.21203/rs.3.rs-3376558/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Jun 10;15(1):4926. doi: 10.1038/s41467-024-49350-6. PMID: 37841835 Free PMC article. Updated. Preprint.
-
Dynamin-dependent entry of Chlamydia trachomatis is sequentially regulated by the effectors TarP and TmeA.Nat Commun. 2024 Jun 10;15(1):4926. doi: 10.1038/s41467-024-49350-6. Nat Commun. 2024. PMID: 38858371 Free PMC article.
-
The emerging complexity of Chlamydia trachomatis interactions with host cells as revealed by molecular genetic approaches.Curr Opin Microbiol. 2023 Aug;74:102330. doi: 10.1016/j.mib.2023.102330. Epub 2023 May 27. Curr Opin Microbiol. 2023. PMID: 37247566 Free PMC article. Review.
-
Contributions of diverse models of the female reproductive tract to the study of Chlamydia trachomatis-host interactions.Curr Opin Microbiol. 2024 Feb;77:102416. doi: 10.1016/j.mib.2023.102416. Epub 2023 Dec 15. Curr Opin Microbiol. 2024. PMID: 38103413 Free PMC article. Review.
-
Pathogenicity and virulence of Chlamydia trachomatis: Insights into host interactions, immune evasion, and intracellular survival.Virulence. 2025 Dec;16(1):2503423. doi: 10.1080/21505594.2025.2503423. Epub 2025 May 15. Virulence. 2025. PMID: 40353442 Free PMC article. Review.
References
-
- Bailly, M., Macaluso, F., Cammer, M., Chan, A., Segall, J. E. and Condeelis, J. S. (1999). Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J. Cell Biol. 145, 331-345. 10.1083/jcb.145.2.331 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

