Preclinical Evaluation of Foamy Virus Vector-Mediated Gene Addition in Human Hematopoietic Stem/Progenitor Cells for Correction of Leukocyte Adhesion Deficiency Type 1
- PMID: 36094106
- PMCID: PMC9808799
- DOI: 10.1089/hum.2022.065
Preclinical Evaluation of Foamy Virus Vector-Mediated Gene Addition in Human Hematopoietic Stem/Progenitor Cells for Correction of Leukocyte Adhesion Deficiency Type 1
Abstract
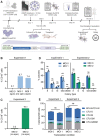
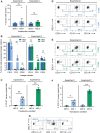
Ex vivo gene therapy procedures targeting hematopoietic stem and progenitor cells (HSPCs) predominantly utilize lentivirus-based vectors for gene transfer. We provide the first pre-clinical evidence of the therapeutic utility of a foamy virus vector (FVV) for the genetic correction of human leukocyte adhesion deficiency type 1 (LAD-1), an inherited primary immunodeficiency resulting from mutation of the β2 integrin common chain, CD18. CD34+ HSPCs isolated from a severely affected LAD-1 patient were transduced under a current good manufacturing practice-compatible protocol with FVV harboring a therapeutic CD18 transgene. LAD-1-associated cellular chemotactic defects were ameliorated in transgene-positive, myeloid-differentiated LAD-1 cells assayed in response to a strong neutrophil chemoattractant in vitro. Xenotransplantation of vector-transduced LAD-1 HSPCs in immunodeficient (NSG) mice resulted in long-term (∼5 months) human cell engraftment within murine bone marrow. Moreover, engrafted LAD-1 myeloid cells displayed in vivo levels of transgene marking previously reported to ameliorate the LAD-1 phenotype in a large animal model of the disease. Vector insertion site analysis revealed a favorable vector integration profile with no overt evidence of genotoxicity. These results coupled with the unique biological features of wild-type foamy virus support the development of FVVs for ex vivo gene therapy of LAD-1.
Keywords: foamy virus; hematopoietic stem and progenitor cells; leukocyte adhesion deficiency type 1; β2 integrin.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency.Mol Ther. 2013 May;21(5):964-72. doi: 10.1038/mt.2013.34. Epub 2013 Mar 26. Mol Ther. 2013. PMID: 23531552 Free PMC article.
-
Retroviral-mediated gene transfer of the leukocyte integrin CD18 into peripheral blood CD34+ cells derived from a patient with leukocyte adhesion deficiency type 1.Blood. 1998 Mar 1;91(5):1520-6. Blood. 1998. PMID: 9473215
-
Treatment of canine leukocyte adhesion deficiency by foamy virus vectors expressing CD18 from a PGK promoter.Gene Ther. 2011 Jun;18(6):553-9. doi: 10.1038/gt.2010.169. Epub 2011 Jan 13. Gene Ther. 2011. PMID: 21228879 Free PMC article.
-
In-Vivo Gene Therapy with Foamy Virus Vectors.Viruses. 2019 Nov 23;11(12):1091. doi: 10.3390/v11121091. Viruses. 2019. PMID: 31771194 Free PMC article. Review.
-
Gene therapy for leukocyte adhesion deficiency.Curr Opin Mol Ther. 2000 Aug;2(4):383-8. Curr Opin Mol Ther. 2000. PMID: 11249768 Review.
Cited by
-
Progress in the field of hematopoietic stem cell-based therapies for inborn errors of immunity.Curr Opin Pediatr. 2023 Dec 1;35(6):663-670. doi: 10.1097/MOP.0000000000001292. Epub 2023 Sep 21. Curr Opin Pediatr. 2023. PMID: 37732933 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

