Siderophore Immunization Restricted Colonization of Adherent-Invasive Escherichia coli and Ameliorated Experimental Colitis
- PMID: 36094114
- PMCID: PMC9600343
- DOI: 10.1128/mbio.02184-22
Siderophore Immunization Restricted Colonization of Adherent-Invasive Escherichia coli and Ameliorated Experimental Colitis
Abstract
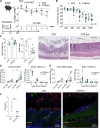
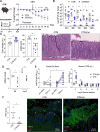
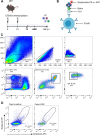
Inflammatory bowel diseases (IBD) are characterized by chronic inflammation of the gastrointestinal tract and profound alterations to the gut microbiome. Adherent-invasive Escherichia coli (AIEC) is a mucosa-associated pathobiont that colonizes the gut of patients with Crohn's disease, a form of IBD. Because AIEC exacerbates gut inflammation, strategies to reduce the AIEC bloom during colitis are highly desirable. To thrive in the inflamed gut, Enterobacteriaceae acquire the essential metal nutrient iron by producing and releasing siderophores. Here, we implemented an immunization-based strategy to target the siderophores enterobactin and its glucosylated derivative salmochelin to reduce the AIEC bloom in the inflamed gut. Using chemical (dextran sulfate sodium) and genetic (Il10-/- mice) IBD mouse models, we showed that immunization with enterobactin conjugated to the mucosal adjuvant cholera toxin subunit B potently elicited mucosal and serum antibodies against these siderophores. Siderophore-immunized mice exhibited lower AIEC gut colonization, diminished AIEC association with the gut mucosa, and reduced colitis severity. Moreover, Peyer's patches and the colonic lamina propria harbored enterobactin-specific B cells that could be identified by flow cytometry. The beneficial effect of siderophore immunization was primarily B cell-dependent because immunized muMT-/- mice, which lack mature B lymphocytes, were not protected during AIEC infection. Collectively, our study identified siderophores as a potential therapeutic target to reduce AIEC colonization and its association with the gut mucosa, which ultimately may reduce colitis exacerbation. Moreover, this work provides the foundation for developing monoclonal antibodies against siderophores, which could provide a narrow-spectrum strategy to target the AIEC bloom in Crohn's disease patients. IMPORTANCE Adherent-invasive Escherichia coli (AIEC) is abnormally prevalent in patients with ileal Crohn's disease and exacerbates intestinal inflammation, but treatment strategies that selectively target AIEC are unavailable. Iron is an essential micronutrient for most living organisms, and bacterial pathogens have evolved sophisticated strategies to capture iron from the host environment. AIEC produces siderophores, small, secreted molecules with a high affinity for iron. Here, we showed that immunization to elicit antibodies against siderophores promoted a reduction of the AIEC bloom, interfered with AIEC association with the mucosa, and mitigated colitis in experimental mouse models. We also established a flow cytometry-based approach to visualize and isolate siderophore-specific B cells, a prerequisite for engineering monoclonal antibodies against these molecules. Together, this work could lead to a more selective and antibiotic-sparing strategy to target AIEC in Crohn's disease patients.
Keywords: Escherichia coli; gut inflammation; immunization; inflammatory bowel disease; iron utilization; mucosal immunity; siderophores.
Conflict of interest statement
The authors declare a conflict of interest. E.M.N. and M.R. hold a patent related to this work.
Figures


Similar articles
-
Development of Heptylmannoside-Based Glycoconjugate Antiadhesive Compounds against Adherent-Invasive Escherichia coli Bacteria Associated with Crohn's Disease.mBio. 2015 Nov 17;6(6):e01298-15. doi: 10.1128/mBio.01298-15. mBio. 2015. PMID: 26578673 Free PMC article.
-
Association of Adherent-invasive Escherichia coli with severe Gut Mucosal dysbiosis in Hong Kong Chinese population with Crohn's disease.Gut Microbes. 2021 Jan-Dec;13(1):1994833. doi: 10.1080/19490976.2021.1994833. Gut Microbes. 2021. PMID: 34812117 Free PMC article.
-
A consortia of clinical E. coli strains with distinct in vitro adherent/invasive properties establish their own co-colonization niche and shape the intestinal microbiota in inflammation-susceptible mice.Microbiome. 2023 Dec 20;11(1):277. doi: 10.1186/s40168-023-01710-y. Microbiome. 2023. PMID: 38124090 Free PMC article.
-
The multifaceted virulence of adherent-invasive Escherichia coli.Gut Microbes. 2023 Jan-Dec;15(1):2172669. doi: 10.1080/19490976.2023.2172669. Gut Microbes. 2023. PMID: 36740845 Free PMC article. Review.
-
Adherent-Invasive E. coli: Update on the Lifestyle of a Troublemaker in Crohn's Disease.Int J Mol Sci. 2020 May 25;21(10):3734. doi: 10.3390/ijms21103734. Int J Mol Sci. 2020. PMID: 32466328 Free PMC article. Review.
Cited by
-
Exploring the Antibacterial Activity and Cellular Fates of Enterobactin-Drug Conjugates That Target Gram-Negative Bacterial Pathogens.Acc Chem Res. 2024 Apr 2;57(7):1046-1056. doi: 10.1021/acs.accounts.3c00814. Epub 2024 Mar 14. Acc Chem Res. 2024. PMID: 38483177 Free PMC article.
-
Interplay of m6A RNA methylation and gut microbiota in modulating gut injury.Gut Microbes. 2025 Dec;17(1):2467213. doi: 10.1080/19490976.2025.2467213. Epub 2025 Feb 17. Gut Microbes. 2025. PMID: 39960310 Free PMC article. Review.
-
Categorization of the effects of E. coli LF82 and mutants lacking the chuT and shuU genes on survival, the transcriptome, and metabolome in germ-free honeybee.FEBS Open Bio. 2024 May;14(5):756-770. doi: 10.1002/2211-5463.13776. Epub 2024 Feb 25. FEBS Open Bio. 2024. PMID: 38403884 Free PMC article.
-
Opportunities and challenges of microbial siderophores in the medical field.Appl Microbiol Biotechnol. 2023 Nov;107(22):6751-6759. doi: 10.1007/s00253-023-12742-7. Epub 2023 Sep 27. Appl Microbiol Biotechnol. 2023. PMID: 37755507 Free PMC article. Review.
-
Stratifying macrophages based on their infectious burden identifies novel host targets for intervention during Crohn's disease associated adherent-invasive Escherichia coli infection.Microbiology (Reading). 2024 Jun;170(6):001470. doi: 10.1099/mic.0.001470. Microbiology (Reading). 2024. PMID: 38916198 Free PMC article.
References
-
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar J-P, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. 2012. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124. doi:10.1038/nature11582. - DOI - PMC - PubMed
-
- Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Björklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. 2019. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 567:49–55. doi:10.1038/s41586-019-0992-y. - DOI - PubMed

