Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a Hybrid III Implementation-effectiveness study (CUSTOMIZE)
- PMID: 36094142
- PMCID: PMC9465974
- DOI: 10.1002/jia2.26003
Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a Hybrid III Implementation-effectiveness study (CUSTOMIZE)
Abstract
Introduction: CUSTOMIZE evaluated the implementation of long-acting (LA) cabotegravir + rilpivirine, a novel healthcare provider-administered injectable antiretroviral therapy regimen, in diverse US healthcare settings. Findings from staff-study participants (SSPs) through 12 months of implementation are reported.
Methods: CUSTOMIZE was a phase IIIb, 12-month, single-arm, hybrid III implementation-effectiveness study conducted from July 2019 to October 2020 at eight US clinics of five clinic types: private practice (n = 2), federally qualified health centre (n = 2), university (n = 2), AIDS Healthcare Foundation (n = 2) and health maintenance organization (n = 1). Eligible patient participants received monthly cabotegravir + rilpivirine LA injections after a 1-month oral lead-in. At baseline, month 4 and month 12, SSPs (n = 3 each per clinic), including physicians, nurses or injectors, and administrators, completed quantitative surveys and semi-structured interviews to assess implementation outcomes (acceptability, appropriateness and feasibility of intervention measures), programme sustainability and SSP perceptions of, attitudes towards, and expectations for cabotegravir + rilpivirine LA. Month 12 data collection occurred during the COVID-19 pandemic.
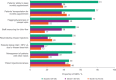
Results: In surveys, SSPs reported high mean total scores for acceptability, appropriateness and feasibility of cabotegravir + rilpivirine LA implementation at baseline (4.43, 4.52 and 4.38 of 5, respectively) and month 12 (4.45, 4.61 and 4.46 of 5, respectively), regardless of clinic type. At month 12, SSPs were positive about the implementation sustainability (mean Program Sustainability Assessment Tool score, 5.83 out of 7). At baseline, SSPs' top concern was patients' ability to maintain monthly appointments (81%); at month 12, 39% had this concern. The proportion of SSPs reporting patient injection pain or soreness as a barrier was consistent at month 12 versus baseline (48% vs. 46%). Most (78%) SSPs reported optimal implementation of cabotegravir + rilpivirine LA in their clinics was achieved in 1-3 months. In interviews, SSP-reported strategies for successful implementation included teamwork, using a web-based treatment planner and having a designated person to track appointment scheduling. In month 12 interviews, SSP-reported structural changes needed for implementation included changing clinic hours and purchasing refrigerators.
Conclusions: In CUSTOMIZE, cabotegravir + rilpivirine LA was successfully implemented across a range of US healthcare settings. Barriers were mitigated with minor process adjustments.
Trial registration: ClinicalTrials.gov NCT04001803.
Keywords: HIV-1; acceptability; antiretroviral therapy; appropriateness; feasibility; sustainability.
© 2022 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
MC, CG, MD, RD, TW, DM and WS are employees of ViiV Healthcare and may own stock in GSK. WW and YW are employees of and may own stock in GSK. LS is an employee of Evidera, which receives funding from ViiV Healthcare for their work. MW serves on the Janssen HIV Prophylactic Vaccine Advisory Board. GS reports grants and personal fees from Janssen, ViiV Healthcare and Merck, and grants from Gilead Sciences. LM reports grants and personal fees from Gilead Sciences, GSK, ViiV Healthcare and MSD, and grants from Janssen, Visby Medical, ThaiMed, Evofem Biosciences, SpeeDx Pty Ltd and Lupin Pharmaceutical. BT owns stock in ViiV Healthcare. JF has served as a principal investigator for ViiV Healthcare. PB has nothing to disclose.
Figures



References
-
- Cabenuva [package insert] . Research Triangle Park, NC: ViiV Healthcare; 2021.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents [Internet]. [Cited 2022 Jan 5]. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Ad....
-
- Orkin C, Arasteh K, Górgolas Hernández‐Mora M, Pokrovsky V, Overton ET, Girard P‐M, et al. Long‐acting cabotegravir and rilpivirine after oral induction for HIV‐1 infection. N Engl J Med. 2020;382(12):1124‐35. - PubMed
-
- Swindells S, Andrade‐Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long‐acting cabotegravir and rilpivirine for maintenance of HIV‐1 suppression. N Engl J Med. 2020;382(12):1112‐23. - PubMed
-
- Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Long‐acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV‐1 infection (ATLAS‐2M), 48‐week results: a randomised, multicentre, open‐label, phase 3b, non‐inferiority study. Lancet. 2021;396(10267):1994‐2005. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

