The acid ceramidase/ceramide axis controls parasitemia in Plasmodium yoelii-infected mice by regulating erythropoiesis
- PMID: 36094170
- PMCID: PMC9499531
- DOI: 10.7554/eLife.77975
The acid ceramidase/ceramide axis controls parasitemia in Plasmodium yoelii-infected mice by regulating erythropoiesis
Abstract
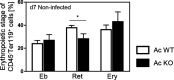
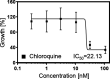
Acid ceramidase (Ac) is part of the sphingolipid metabolism and responsible for the degradation of ceramide. As bioactive molecule, ceramide is involved in the regulation of many cellular processes. However, the impact of cell-intrinsic Ac activity and ceramide on the course of Plasmodium infection remains elusive. Here, we use Ac-deficient mice with ubiquitously increased ceramide levels to elucidate the role of endogenous Ac activity in a murine malaria model. Interestingly, ablation of Ac leads to alleviated parasitemia associated with decreased T cell responses in the early phase of Plasmodium yoelii infection. Mechanistically, we identified dysregulated erythropoiesis with reduced numbers of reticulocytes, the preferred host cells of P. yoelii, in Ac-deficient mice. Furthermore, we demonstrate that administration of the Ac inhibitor carmofur to wildtype mice has similar effects on P. yoelii infection and erythropoiesis. Notably, therapeutic carmofur treatment after manifestation of P. yoelii infection is efficient in reducing parasitemia. Hence, our results provide evidence for the involvement of Ac and ceramide in controlling P. yoelii infection by regulating red blood cell development.
Keywords: ceramide; infectious disease; malaria; microbiology; mouse; sphingolipids.
© 2022, Günther et al.
Conflict of interest statement
AG, MH, HA, FS, YV, BK, KM, KL, EG, JB, AW, WH No competing interests declared
Figures







Similar articles
-
Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte.Blood. 2001 Jun 15;97(12):3966-71. doi: 10.1182/blood.v97.12.3966. Blood. 2001. PMID: 11389041
-
Amitriptyline inhibits Plasmodium development in infected red blood cells by modulating sphingolipid metabolism and glucose uptake.Biomed Pharmacother. 2025 Aug;189:118331. doi: 10.1016/j.biopha.2025.118331. Epub 2025 Jul 8. Biomed Pharmacother. 2025. PMID: 40633203
-
The dynamic landscape of parasitemia dependent intestinal microbiota shifting and the correlated gut transcriptome during Plasmodium yoelii infection.Microbiol Res. 2022 May;258:126994. doi: 10.1016/j.micres.2022.126994. Epub 2022 Feb 19. Microbiol Res. 2022. PMID: 35220138
-
Antimalarial efficacy, cytotoxicity, and genotoxicity of methanolic stem bark extract from Hintonia latiflora in a Plasmodium yoelii yoelii lethal murine malaria model.Parasitol Res. 2014 Apr;113(4):1529-36. doi: 10.1007/s00436-014-3797-9. Epub 2014 Feb 19. Parasitol Res. 2014. PMID: 24549754
-
T Cell-Specific Overexpression of Acid Sphingomyelinase Results in Elevated T Cell Activation and Reduced Parasitemia During Plasmodium yoelii Infection.Front Immunol. 2019 May 31;10:1225. doi: 10.3389/fimmu.2019.01225. eCollection 2019. Front Immunol. 2019. PMID: 31214184 Free PMC article.
Cited by
-
Host sphingolipids support Plasmodium berghei liver stage development.mBio. 2025 Aug 13;16(8):e0167525. doi: 10.1128/mbio.01675-25. Epub 2025 Jul 21. mBio. 2025. PMID: 40689642 Free PMC article.
-
Acid ceramidase expression reduces IFNγ secretion by mouse CD4+ T cells and is crucial for maintaining B-cell numbers in mice.Front Immunol. 2024 Jun 11;15:1309846. doi: 10.3389/fimmu.2024.1309846. eCollection 2024. Front Immunol. 2024. PMID: 38919612 Free PMC article.
References
-
- Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ, Treatman C, Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Research. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

