A Serotype-Specific and Multiplex PCR Method for Whole-Genome Sequencing of Dengue Virus Directly from Clinical Samples
- PMID: 36094197
- PMCID: PMC9602986
- DOI: 10.1128/spectrum.01210-22
A Serotype-Specific and Multiplex PCR Method for Whole-Genome Sequencing of Dengue Virus Directly from Clinical Samples
Abstract
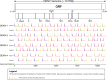
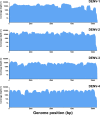
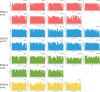
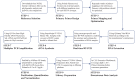
Dengue virus (DENV) is the most globally prevalent member of the genus Flavivirus in the family Flaviviridae, which can be classified into four serotypes. Historically, molecular epidemiological studies of DENV depended on E gene sequencing. The development of next-generation sequencing (NGS) allowed its application to viral whole-genome sequencing (WGS). In this study, we report the improvement of the existing WGS process for DENV by optimizing the primer design procedure, designing serotype-specific primer panels and reducing the sizes of amplicons. A total of 31 DENV-positive serum samples belonging to 4 serotypes and 9 genotypes of DENV were involved in the validation of the primer panels. The threshold cycle (CT) values of these samples ranged from 23.91 to 35.11. The validation results showed that the length of consensus sequences generated at a coverage depth of 20× or more ranged from 10,370 to 10,672 bp, with 100.00% coverage of the open reading frames and 97.34% to 99.52% coverage of the DENV genome. The amplification efficiency varied across amplicons, genotypes, and serotypes of DENVs. These results indicate that the serotype-specific primer panels allow users to obtain the whole genome of DENV directly from clinical samples, providing a universal, rapid, and effective tool for the integration of genomics with dengue surveillance. IMPORTANCE Dengue virus (DENV) is becoming the most globally prevalent arbovirus. The number of people living under the threat of DENV is increasing year by year. With the development of next-generation sequencing (NGS) technology, whole-genome sequencing (WGS) has been more and more widely used in infectious disease surveillance and molecular epidemiological studies. DENV population sequencing by NGS can increase our understanding of the changing epidemiology and evolution of the DENV genome at the molecular level, which demands universal primer panels and combination with NGS platforms. Multiplex PCR with a short-amplicon approach proved superior for amplifying viral genomes from clinical samples, particularly when the viral RNA was present at low concentrations. Additionally, DENV are known for their genetic diversity within serotype groups and geographical regions, so the primer panels we designed focused on universality, which would be useful in future local DENV outbreaks.
Keywords: dengue; dengue virus; multiplex PCR; next-generation sequencing; whole-genome sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Assessment of a multiplex PCR and Nanopore-based method for dengue virus sequencing in Indonesia.Virol J. 2020 Feb 13;17(1):24. doi: 10.1186/s12985-020-1294-6. Virol J. 2020. PMID: 32054488 Free PMC article.
-
Detection of genotype-1 of dengue virus serotype 3 for the first time and complete genome analysis of dengue viruses during the 2018 epidemic in Mandalay, Upper Myanmar.PLoS One. 2021 Jun 4;16(6):e0251314. doi: 10.1371/journal.pone.0251314. eCollection 2021. PLoS One. 2021. PMID: 34086703 Free PMC article.
-
DengueSeq: a pan-serotype whole genome amplicon sequencing protocol for dengue virus.BMC Genomics. 2024 May 1;25(1):433. doi: 10.1186/s12864-024-10350-x. BMC Genomics. 2024. PMID: 38693476 Free PMC article.
-
Unraveling Dengue Virus Diversity in Asia: An Epidemiological Study through Genetic Sequences and Phylogenetic Analysis.Viruses. 2024 Jun 28;16(7):1046. doi: 10.3390/v16071046. Viruses. 2024. PMID: 39066210 Free PMC article. Review.
-
The Epidemiological Impact of Dengue in Colombia: A Systematic Review.Am J Trop Med Hyg. 2024 Oct 29;112(1):182-188. doi: 10.4269/ajtmh.23-0907. Print 2025 Jan 8. Am J Trop Med Hyg. 2024. PMID: 39471503 Free PMC article.
Cited by
-
DengueSeq: A pan-serotype whole genome amplicon sequencing protocol for dengue virus.medRxiv [Preprint]. 2023 Oct 13:2023.10.13.23296997. doi: 10.1101/2023.10.13.23296997. medRxiv. 2023. Update in: BMC Genomics. 2024 May 1;25(1):433. doi: 10.1186/s12864-024-10350-x. PMID: 37873191 Free PMC article. Updated. Preprint.
-
Complete genome sequence of dengue virus serotype 2 obtained from Chattogram, Bangladesh.Microbiol Resour Announc. 2025 May 8;14(5):e0002325. doi: 10.1128/mra.00023-25. Epub 2025 Apr 17. Microbiol Resour Announc. 2025. PMID: 40243309 Free PMC article.
-
Outbreaks of autochthonous Dengue in Lazio region, Italy, August to September 2023: preliminary investigation.Euro Surveill. 2023 Nov;28(44):2300552. doi: 10.2807/1560-7917.ES.2023.28.44.2300552. Euro Surveill. 2023. PMID: 37917030 Free PMC article.
-
Clinical value of macrogenome next-generation sequencing on infections.Open Life Sci. 2024 Sep 9;19(1):20220938. doi: 10.1515/biol-2022-0938. eCollection 2024. Open Life Sci. 2024. PMID: 39290502 Free PMC article.
-
Spatiotemporal dispersion of DENV-1 genotype V in Western Colombia.Virus Evol. 2025 Apr 16;11(1):veaf018. doi: 10.1093/ve/veaf018. eCollection 2025. Virus Evol. 2025. PMID: 40395613 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

