Kin Discrimination Modifies Strain Distribution, Spatial Segregation, and Incorporation of Extracellular Matrix Polysaccharide Mutants of Bacillus subtilis Strains into Mixed Floating Biofilms
- PMID: 36094206
- PMCID: PMC9499035
- DOI: 10.1128/aem.00871-22
Kin Discrimination Modifies Strain Distribution, Spatial Segregation, and Incorporation of Extracellular Matrix Polysaccharide Mutants of Bacillus subtilis Strains into Mixed Floating Biofilms
Abstract
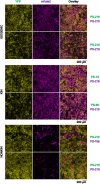
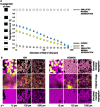
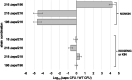
Microorganisms in nature form multicellular groups called biofilms. In biofilms, bacteria embedded in the extracellular matrix (ECM) interact intensely due to their proximity. Most studies have investigated genetically homogeneous biofilms, leaving a gap in knowledge on genetically heterogeneous biofilms. Recent insights show that a Gram-positive model bacterium, Bacillus subtilis, discriminates between strains of high (kin) and low (nonkin) genetic similarity, reflected in merging (kin) and boundaries (nonkin) between swarms. However, it is unclear how kinship between interacting strains affects their fitness, the genotype assortment, and incorporation of the mutant lacking the main structural ECM polysaccharide (EpsA-O) into floating biofilms (pellicles). We cultivated Bacillus subtilis strains as mixtures of isogenic, kin, and nonkin strain combinations in the biofilm-promoting minimal medium under static conditions, allowing them to form pellicles. We show that in nonkin pellicles, the dominant strain strongly reduced the frequency of the other strain. Segregation of nonkin mixtures in pellicles increased and invasion of nonkin EpsA-O-deficient mutants into pellicles decreased compared to kin and isogenic floating biofilms. Kin and isogenic strains had comparable relative frequencies in pellicles and showed more homogenous cell mixing. Overall, our results emphasize kin discrimination as a social behavior that shapes strain distribution, spatial segregation, and ECM mutant ability to incorporate into genetically heterogenous biofilms of B. subtilis. IMPORTANCE Biofilm communities have beneficial and harmful effects on human societies in natural, medical, and industrial environments. Bacillus subtilis is a biotechnologically important bacterium that serves as a model for studying biofilms. Recent studies have shown that this species engages in kin discriminatory behavior during swarming, which may have implications for community assembly, thus being of fundamental importance. Effects of kin discrimination on fitness, genotype segregation, and success of extracellular matrix (ECM) polysaccharide (EpsA-O) mutant invasion into biofilms are not well understood. We provide evidence that kin discrimination depends on the antagonism of the dominant strain against nonkin by using environmental strains with determined kin types and integrated fluorescent reporters. Moreover, this antagonism has important implications for genotype segregation and for when the bacteria are mixed with ECM producers. The work advances the understanding of kin-discrimination-dependent bacterial sociality in biofilms and its role in the assembly of multicellular groups.
Keywords: Bacillus subtilis; biofilms; incorporation of extracellular matrix polysaccharide mutant; kin discrimination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bacillus subtilis Protects Public Goods by Extending Kin Discrimination to Closely Related Species.mBio. 2017 Jul 5;8(4):e00723-17. doi: 10.1128/mBio.00723-17. mBio. 2017. PMID: 28679746 Free PMC article.
-
Kin discrimination between sympatric Bacillus subtilis isolates.Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):14042-7. doi: 10.1073/pnas.1512671112. Epub 2015 Oct 5. Proc Natl Acad Sci U S A. 2015. PMID: 26438858 Free PMC article.
-
Kin discrimination drives territorial exclusion during Bacillus subtilis swarming and restrains exploitation of surfactin.ISME J. 2022 Mar;16(3):833-841. doi: 10.1038/s41396-021-01124-4. Epub 2021 Oct 14. ISME J. 2022. PMID: 34650232 Free PMC article.
-
Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms.Mol Microbiol. 2014 Aug;93(4):587-98. doi: 10.1111/mmi.12697. Epub 2014 Jul 18. Mol Microbiol. 2014. PMID: 24988880 Free PMC article. Review.
-
Social behaviours by Bacillus subtilis: quorum sensing, kin discrimination and beyond.Mol Microbiol. 2018 Dec;110(6):863-878. doi: 10.1111/mmi.14127. Epub 2018 Nov 1. Mol Microbiol. 2018. PMID: 30218468 Free PMC article. Review.
Cited by
-
Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation.Micromachines (Basel). 2024 Aug 15;15(8):1037. doi: 10.3390/mi15081037. Micromachines (Basel). 2024. PMID: 39203688 Free PMC article.
-
Bacterial social interactions in synthetic Bacillus consortia enhance plant growth.Imeta. 2025 Jun 8;4(4):e70053. doi: 10.1002/imt2.70053. eCollection 2025 Aug. Imeta. 2025. PMID: 40860443 Free PMC article.
-
Bacillus subtilis EpsA-O: A novel exopolysaccharide structure acting as an efficient adhesive in biofilms.NPJ Biofilms Microbiomes. 2024 Oct 2;10(1):98. doi: 10.1038/s41522-024-00555-z. NPJ Biofilms Microbiomes. 2024. PMID: 39358392 Free PMC article.
-
Bacillus subtilis Intraspecies Interactions Shape Probiotic Activity Against Salmonella Typhimurium.Microb Biotechnol. 2024 Dec;17(12):e70065. doi: 10.1111/1751-7915.70065. Microb Biotechnol. 2024. PMID: 39718437 Free PMC article.
-
Nutrient Availability and Biofilm Polysaccharide Shape the Bacillaene-Dependent Antagonism of Bacillus subtilis against Salmonella Typhimurium.Microbiol Spectr. 2022 Dec 21;10(6):e0183622. doi: 10.1128/spectrum.01836-22. Epub 2022 Nov 7. Microbiol Spectr. 2022. PMID: 36342318 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

