Reovirus Efficiently Reassorts Genome Segments during Coinfection and Superinfection
- PMID: 36094315
- PMCID: PMC9517712
- DOI: 10.1128/jvi.00910-22
Reovirus Efficiently Reassorts Genome Segments during Coinfection and Superinfection
Abstract
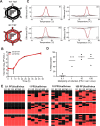
Reassortment, or genome segment exchange, increases diversity among viruses with segmented genomes. Previous studies on the limitations of reassortment have largely focused on parental incompatibilities that restrict generation of viable progeny. However, less is known about whether factors intrinsic to virus replication influence reassortment. Mammalian orthoreovirus (reovirus) encapsidates a segmented, double-stranded RNA (dsRNA) genome, replicates within cytoplasmic factories, and is susceptible to host antiviral responses. We sought to elucidate the influence of infection multiplicity, timing, and compartmentalized replication on reovirus reassortment in the absence of parental incompatibilities. We used an established post-PCR genotyping method to quantify reassortment frequency between wild-type and genetically barcoded type 3 reoviruses. Consistent with published findings, we found that reassortment increased with infection multiplicity until reaching a peak of efficient genome segment exchange during simultaneous coinfection. However, reassortment frequency exhibited a substantial decease with increasing time to superinfection, which strongly correlated with viral transcript abundance. We hypothesized that physical sequestration of viral transcripts within distinct virus factories or superinfection exclusion also could influence reassortment frequency during superinfection. Imaging revealed that transcripts from both wild-type and barcoded viruses frequently co-occupied factories, with superinfection time delays up to 16 h. Additionally, primary infection progressively dampened superinfecting virus transcript levels with greater time delay to superinfection. Thus, in the absence of parental incompatibilities and with short times to superinfection, reovirus reassortment proceeds efficiently and is largely unaffected by compartmentalization of replication and superinfection exclusion. However, reassortment may be limited by superinfection exclusion with greater time delays to superinfection. IMPORTANCE Reassortment, or genome segment exchange between viruses, can generate novel virus genotypes and pandemic virus strains. For viruses to reassort their genome segments, they must replicate within the same physical space by coinfecting the same host cell. Even after entry into the host cell, many viruses with segmented genomes synthesize new virus transcripts and assemble and package their genomes within cytoplasmic replication compartments. Additionally, some viruses can interfere with subsequent infection of the same host or cell. However, spatial and temporal influences on reassortment are only beginning to be explored. We found that infection multiplicity and transcript abundance are important drivers of reassortment during coinfection and superinfection, respectively, for reovirus, which has a segmented, double-stranded RNA genome. We also provide evidence that compartmentalization of transcription and packaging is unlikely to influence reassortment, but the length of time between primary and subsequent reovirus infection can alter reassortment frequency.
Keywords: coinfection; double-stranded RNA virus; reassortment; reovirus; superinfection; superinfection exclusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

