Current updates on generations, approvals, and clinical trials of CAR T-cell therapy
- PMID: 36094837
- PMCID: PMC9746433
- DOI: 10.1080/21645515.2022.2114254
Current updates on generations, approvals, and clinical trials of CAR T-cell therapy
Abstract
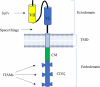
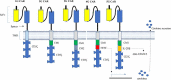
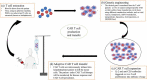
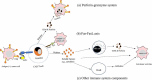
Chimeric antigen receptor (CAR) T-cell therapy is a novel, customized immunotherapy that is considered a 'living' and self-replicating drug to treat cancer, sometimes resulting in a complete cure. CAR T-cells are manufactured through genetic engineering of T-cells by equipping them with CARs to detect and target antigen-expressing cancer cells. CAR is designed to have an ectodomain extracellularly, a transmembrane domain spanning the cell membrane, and an endodomain intracellularly. Since its first discovery, the CAR structure has evolved greatly, from the first generation to the fifth generation, to offer new therapeutic alternatives for cancer patients. This treatment has achieved long-term and curative therapeutic efficacy in multiple blood malignancies that nowadays profoundly change the treatment landscape of lymphoma, leukemia, and multiple myeloma. But CART-cell therapy is associated with several hurdles, such as limited therapeutic efficacy, little effect on solid tumors, adverse effects, expensive cost, and feasibility issues, hindering its broader implications.
Keywords: CAR; CAR T-cell therapy; approval; clinical trials; generations; structure.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
