A Bifurcated Multicomponent Synthesis Approach to Polycyclic Quinazolinones
- PMID: 36095044
- PMCID: PMC9552225
- DOI: 10.1021/acs.joc.2c01561
A Bifurcated Multicomponent Synthesis Approach to Polycyclic Quinazolinones
Abstract
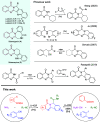
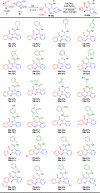
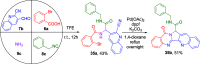
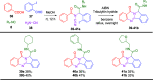
The rapid synthesis of diverse substituted polycyclic quinazolinones was achieved by two orthogonal Ugi four-component reaction (Ugi-4CR)-based protocols: the first two-step approach via an ammonia-Ugi-4CR followed by palladium-catalyzed annulation; in the second approach, cyanamide was used unprecedently as an amine component in Ugi-4CR followed by an AIBN/tributyltin hydride-induced radical reaction. Like no other method, MCR and cyclization could efficiently construct many biologically interesting compounds with tailored properties in very few steps.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
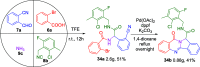
References
-
- Hameed A.; Al-Rashida M.; Uroos M.; Ali S. A.; Arshia; Ishtiaq M.; Khan K. M. Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. 10.1080/13543776.2018.1432596. - DOI - PubMed
- Auti P. S.; George G.; Paul A. T. Recent advances in the pharmacological diversification of quinazoline/ quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. 10.1039/D0RA06642G. - DOI - PMC - PubMed
- Biological activity of quinazolinones; Radwan A. A., Alanazi F. K. Eds.; Books on Demand: London, 2020.
-
- Shakhidoyatov K. M.; Elmuradov B. Z. Tricyclic quinazoline alkaloids: isolation, synthesis, chemical modification, and biological activity. Chem. Nat. Compd. 2014, 50, 781–800. 10.1007/s10600-014-1086-6. - DOI
- Cagir A.; Jones S. H.; Gao R.; Eisenhauer B. M.; Hecht S. M. Luotonin A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. 10.1021/ja0368857. - DOI - PubMed
- Joshi B. K.; Gloer J. B.; Wicklow D. T.; Dowd P. F. Sclerotigenin: a new antiinsectan benzodiazepine from the sclerotia of Penicillium sclerotigenum. J. Nat. Prod. 1999, 62, 650–652. 10.1021/np980511n. - DOI - PubMed
- Tian K. M.; Li J. J.; Xu S. W. Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 2019, 141, 541–550. 10.1016/j.phrs.2018.12.019. - DOI - PubMed
-
- Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. - DOI - PubMed
- Dömling A.; Ugi I. Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. - DOI - PubMed
-
- Dömling A.; Wang W.; Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. - DOI - PMC - PubMed
- Younus H. A.; Al-Rashida M.; Hameed A.; Uroos M.; Salar U.; Rana S.; Khan K. M. Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010-2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. 10.1080/13543776.2021.1858797. - DOI - PubMed
- Rotstein B. H.; Zaretsky S.; Rai V.; Yudin A. K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. 10.1021/cr400615v. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

