Novel Isobaric Tagging Reagent Enabled Multiplex Quantitative Glycoproteomics via Electron-Transfer/Higher-Energy Collisional Dissociation (EThcD) Mass Spectrometry
- PMID: 36095095
- PMCID: PMC10160164
- DOI: 10.1021/jasms.2c00177
Novel Isobaric Tagging Reagent Enabled Multiplex Quantitative Glycoproteomics via Electron-Transfer/Higher-Energy Collisional Dissociation (EThcD) Mass Spectrometry
Abstract
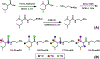
Protein glycosylation, covalent attachment of carbohydrates to polypeptide chains, is a highly important post-translational modification involved in many essential physiological processes. Comprehensive site-specific and quantitative analysis is crucial for revealing the diverse functions and dynamics of glycosylation. To characterize intact glycopeptides, mass spectrometry (MS)-based glycoproteomics employs versatile fragmentation methods, among which electron-transfer/higher-energy collision dissociation (EThcD) has gained great popularity. However, the inherent limitation of EThcD in fragmenting low-charge ions has prevented its widespread applications. Furthermore, there is a need to develop a high-throughput strategy for comparative glycoproteomics with a large cohort of samples. Herein, we developed isobaric N,N-dimethyl leucine-derivatized ethylenediamine (DiLeuEN) tags to increase the charge states of glycopeptides, thereby improving the fragmentation efficiency and allowing for in-depth intact glycopeptide analysis, especially for sialoglycopeptides. Moreover, the unique reporter ions of DiLeuEN-labeled glycopeptides generated in tandem MS spectra enable relative quantification of up to four samples in a single analysis, which represents a new high-throughput method for quantitative glycoproteomics.
Keywords: DiLeuEN; EThcD; isobaric labeling; mass spectrometry; quantitative glycoproteomics.
Figures
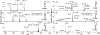

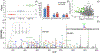
References
-
- Ohtsubo K; Marth JD, Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126 (5), 855–867. - PubMed
-
- Ungar D, Golgi linked protein glycosylation and associated diseases. Semin. Cell Dev. Biol 2009, 20 (7), 762–769. - PubMed
-
- Gudelj I; Lauc G, Protein N-Glycosylation in Cardiovascular Diseases and Related Risk Factors. Curr. Cardiovasc. Risk Rep 2018, 12 (6).
-
- Kristic J; Lauc G, Ubiquitous Importance of Protein Glycosylation. In High-Throughput Glycomics and Glycoproteomics: Methods and Protocols, Lauc G; Wuhrer M, Eds. 2017; Vol. 1503, pp 1–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

