Phosphatidylserine orchestrates Myomerger membrane insertions to drive myoblast fusion
- PMID: 36095199
- PMCID: PMC9499509
- DOI: 10.1073/pnas.2202490119
Phosphatidylserine orchestrates Myomerger membrane insertions to drive myoblast fusion
Abstract
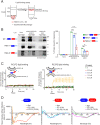
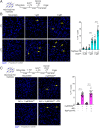
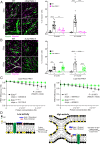
Muscle cell fusion is a multistep process where the final step of the reaction drives progression beyond early hemifusion events to complete fusion. This step requires activity of the muscle-specific fusogen Myomerger, a single-pass transmembrane protein containing 84 amino acids with an ectodomain that includes two α-helices. Previous studies have demonstrated that Myomerger acts by destabilizing membranes through generation of elastic stresses in the outer leaflet of the plasma membrane. An obvious question is how such destabilizing activity might be regulated to avoid membrane and cellular damage, and how the two juxtaposed helices cooperate in fusion. Using cellular fusion assays and in vitro liposome assays, we report that the two helices possess unique characteristics, both of which are needed for full activity of the protein. We demonstrate that externalized phosphatidylserine (PS), a lipid previously implicated in myoblast fusion, has a determinant role in the regulation of Myomerger activity. The membrane-proximal, amphipathic Helix-1 is normally disordered and its α-helical structure is induced by PS, making membrane interactions more efficacious. The distal, more hydrophobic Helix-2 is intrinsically ordered, possesses an ability to insert into membranes, and augments the membrane-stressing effects of Helix-1. These data reveal that Myomerger fusogenic activity is an exquisitely orchestrated event involving its two ectodomain helices, which are controlled by membrane lipid composition, providing an explanation as to how its membrane-stressing activity is spatially and temporally regulated during the final step of myoblast fusion.
Keywords: Myomerger/Myomixer; membrane fusion; myoblast fusion; phosphatidylserine.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Schejter E. D., Myoblast fusion: Experimental systems and cellular mechanisms. Semin. Cell Dev. Biol. 60, 112–120 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

