Targeting Macrophages with CAR T Cells Delays Solid Tumor Progression and Enhances Antitumor Immunity
- PMID: 36095236
- PMCID: PMC10704925
- DOI: 10.1158/2326-6066.CIR-21-1075
Targeting Macrophages with CAR T Cells Delays Solid Tumor Progression and Enhances Antitumor Immunity
Abstract
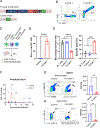
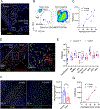
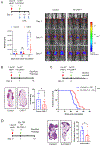
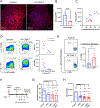
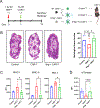
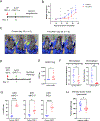
Tumor-associated macrophages (TAM) are one of the most abundant cell types in many solid tumors and typically exert protumor effects. This has led to an interest in macrophage-depleting agents for cancer therapy, but approaches developed to date have had limited success in clinical trials. Here, we report the development of a strategy for TAM depletion in mouse solid tumor models using chimeric antigen receptor (CAR) T cells targeting the macrophage marker F4/80 (F4.CAR-T). F4.CAR-T cells effectively killed macrophages in vitro and in vivo without toxicity. When injected into mice bearing orthotopic lung tumors, F4.CAR-T cells infiltrated tumor lesions and delayed tumor growth comparably with PD-1 blockade, and significantly extended mouse survival. Antitumor effects were mediated by F4.CAR-T-produced IFNγ, which promoted upregulation of MHC molecules on cancer cells and tumor-infiltrating myeloid cells. Notably, F4.CAR-T promoted expansion of endogenous CD8 T cells specific for tumor-associated antigen and led to immune editing of highly antigenic tumor cell clones. Antitumor impact was also observed in mouse models of ovarian and pancreatic cancer. These studies provide proof of principle to support CAR T-cell targeting of TAMs as a means to enhance antitumor immunity.
©2022 American Association for Cancer Research.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures
References
-
- Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity. 2020;52:17–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

