Effective treatment of low-risk acute GVHD with itacitinib monotherapy
- PMID: 36095841
- PMCID: PMC9936304
- DOI: 10.1182/blood.2022017442
Effective treatment of low-risk acute GVHD with itacitinib monotherapy
Abstract
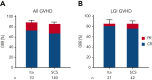
The standard primary treatment for acute graft-versus-host disease (GVHD) requires prolonged, high-dose systemic corticosteroids (SCSs) that delay reconstitution of the immune system. We used validated clinical and biomarker staging criteria to identify a group of patients with low-risk (LR) GVHD that is very likely to respond to SCS. We hypothesized that itacitinib, a selective JAK1 inhibitor, would effectively treat LR GVHD without SCS. We treated 70 patients with LR GVHD in a multicenter, phase 2 trial (NCT03846479) with 28 days of itacitinib 200 mg/d (responders could receive a second 28-day cycle), and we compared their outcomes to those of 140 contemporaneous, matched control patients treated with SCSs. More patients responded to itacitinib within 7 days (81% vs 66%, P = .02), and response rates at day 28 were very high for both groups (89% vs 86%, P = .67), with few symptomatic flares (11% vs 12%, P = .88). Fewer itacitinib-treated patients developed a serious infection within 90 days (27% vs 42%, P = .04) due to fewer viral and fungal infections. Grade ≥3 cytopenias were similar between groups except for less severe leukopenia with itacitinib (16% vs 31%, P = .02). No other grade ≥3 adverse events occurred in >10% of itacitinib-treated patients. There were no significant differences between groups at 1 year for nonrelapse mortality (4% vs 11%, P = .21), relapse (18% vs 21%, P = .64), chronic GVHD (28% vs 33%, P = .33), or survival (88% vs 80%, P = .11). Itacitinib monotherapy seems to be a safe and effective alternative to SCS treatment for LR GVHD and deserves further investigation.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.E. received consulting fees from Kadmon. A.A. received research funding from Incyte. M.M.A.M. received consulting fees and research funding from Incyte. Z.D. received research support from Incyte, Regimmune, and Taiho Oncology and consulting fees from Syndax Pharmaceuticals, Kadmon, Omeros, Incyte, and MorphoSys. C.L.K. received consulting fees from Horizon Therapeutics. F.A. received research funding from Mallinckrodt/Therakos and consulting fees from Celgene/BMS, Gilead, Janssen, Mallinckrodt/Therakos, Medac, Miltenyi Biomedicine, Novartis, and Takeda. U.Ö. is now employed by Eli Lilly and Company. M.Q. received consulting fees from Jazz Pharmaceuticals, Novartis, and Vertex. R.R. received research funding from Atara Biotherapeutics, Gilead Science, J&J, Immatics, Incyte, Pharmacyclics, Precision Biosciences, Shire, and Takeda; consulting fees from Atara Biotherapeutics, Bristol-Myers Squibb, Gilead Sciences, Jasper, Novartis, Regeneron, Synthekine and Tscan; and has an expert witness role with Bayer. Y.-B.C. received consulting fees from Actimium, Celularity, Equillium, Gamida Cell, Incyte, Jasper, and Novartis. J.L.M.F. received research support from Equillium, Genentech, Incyte, and Mesoblast and consulting fees from Alexion, Bo Fu Rui, Equillium, Eurofins Viracor, Nimbus Discovery, Physicians Education Resource, and Xenikos. J.E.L. received research support from Biogen, Equillium, Incyte, MaaT Pharma, and Mesoblast and consulting fees from Bluebird Bio, Equillium, Jazz, Mallinckrodt/Therakos, Mesoblast, and X4 Pharmaceuticals. U.Ö., J.L.M.F., and J.E.L. are coinventors on a GVHD patent and receive royalties. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Progress in risk-adapted acute GVHD therapy.Blood. 2023 Feb 2;141(5):443-444. doi: 10.1182/blood.2022018021. Blood. 2023. PMID: 36729547 No abstract available.
References
-
- Wallace MD, Metzger NL. Optimizing the treatment of steroid-induced hyperglycemia. Ann Pharmacother. 2018;52(1):86–90. - PubMed
-
- Fardet L, Fève B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74(15):1731–1745. - PubMed
-
- Chang C, Greenspan A, Gershwin ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110:102460. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

