Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents
- PMID: 36095849
- PMCID: PMC10562529
- DOI: 10.1182/blood.2022015526
Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents
Abstract
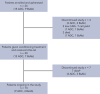
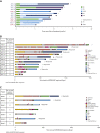
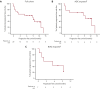
B-cell maturation antigen (BCMA)-targeting therapies, including bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), are promising treatments for multiple myeloma (MM), but disease may progress after their use. CARTITUDE-2 is a phase 2, multicohort study evaluating the safety and efficacy of cilta-cel, an anti-BCMA chimeric antigen receptor T therapy, in various myeloma patient populations. Patients in cohort C progressed despite treatment with a proteasome inhibitor, immunomodulatory drug, anti-CD38 antibody, and noncellular anti-BCMA immunotherapy. A single cilta-cel infusion was given after lymphodepletion. The primary end point was minimal residual disease (MRD) negativity at 10-5. Overall, 20 patients were treated (13 ADC exposed; 7 BsAb exposed; 1 in the ADC group also had prior BsAb exposure). Sixteen (80%) were refractory to prior anti-BCMA therapy. At a median follow-up of 11.3 months (range, 0.6-16.0), 7 of 20 (35%) patients were MRD negative (7 of 10 [70.0%] in the MRD-evaluable subset). Overall response rate (95% confidence interval [CI]) was 60.0% (36.1-80.9). Median duration of response and progression-free survival (95% CI) were 11.5 (7.9-not estimable) and 9.1 (1.5-not estimable) months, respectively. The most common adverse events were hematologic. Cytokine release syndrome occurred in 12 (60%) patients (all grade 1-2); 4 had immune effector cell-associated neurotoxicity syndrome (2 had grade 3-4); none had parkinsonism. Seven (35%) patients died (3 of progressive disease, 4 of adverse events [1 treatment related, 3 unrelated]). Cilta-cel induced favorable responses in patients with relapsed/refractory MM and prior exposure to anti-BCMA treatment who had exhausted other therapies. This trial was registered at www.clinicaltrials.gov as NCT04133636.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.D.C. received research funding from GlaxoSmithKline and Novartis and served as a consultant or advisor for AstraZeneca, Bristol Myers Squibb/Celgene, Genentech/Roche, GlaxoSmithKline, Janssen, Oncopeptides, and Takeda. M.-V.M. received honoraria from or served on the board of directors or on an advisory committee for Amgen, Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, Janssen, Oncopeptides, Pfizer, Regeneron, Roche, Sanofi, Sea-Gen, and Takeda. Y.C.C. received research funding from Amgen, Karyopharm, and Takeda and received honoraria or served as a consultant or advisor to Amgen, GSK, Janssen, Neopharm/Promedico, and Takeda. P.R.-O. received honoraria from or served on speaker bureaus for Bristol Myers Squibb/Celgene, Janssen, Amgen, GlaxoSmithKline, Sanofi, Regeneron, and Oncopeptides. B.P. received research funding from Bristol Myers Squibb/Celgene, Roche, and Sanofi and served as a consultant or advisor for Adaptive, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Kite, Roche, Sanofi, and Takeda. N.W.C.J.v.d.D. received research funding from Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, and Bristol Myers Squibb and served as a consultant or advisor to Janssen, Amgen, Celgene, Bristol Myers Squibb, Takeda, Roche, Novartis, Bayer, Adaptive, and Servier. T.M. received research funding from Janssen. A.S. received research funding from Bristol Myers Squibb, Janssen, GlaxoSmithKline, Regeneron, and Sutro and served as a consultant or advisor to Bristol Myers Squibb, Janssen, and GlaxoSmithKline. K.C.D.B., C.C., J.M.S., H.V., W.D., L.W., M.V., T.R., X.X., P.M., and E.Z. are employed by Janssen. M.A., T.N., and L.P. are employed by Legend Biotech USA Inc. I.A. has received honoraria from or served as a consultant or advisor to GlaxoSmithKline, Janssen, Neopharm/Promedico, Medison, and Gilead. J.S.-M. has served as a consultant, member of board of directors, or on advisory committees for AbbVie, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharp & Dohme, Novartis, Regeneron, Roche, Sanofi, Secura Bio, and Takeda.
Figures

Comment in
-
Sequencing anti-BCMA therapies in myeloma.Blood. 2023 Jan 19;141(3):211-212. doi: 10.1182/blood.2022018157. Blood. 2023. PMID: 36656612 No abstract available.
References
-
- McMillan A, Warcel D, Popat R. Antibody-drug conjugates for multiple myeloma. Expert Opin Biol Ther. 2021;21(7):889–901. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

