Effect of heated tobacco products and traditional cigarettes on pulmonary toxicity and SARS-CoV-2-induced lung injury
- PMID: 36096319
- PMCID: PMC9461237
- DOI: 10.1016/j.tox.2022.153318
Effect of heated tobacco products and traditional cigarettes on pulmonary toxicity and SARS-CoV-2-induced lung injury
Abstract
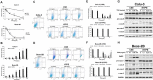
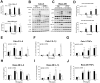
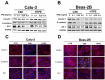
Cigarette smoke (CS) significantly contributes to the development of chronic obstructive pulmonary disease (COPD). Heated tobacco products (HTPs), newly developed cigarette products, have been proposed as an alternative for safe cigarette smoking. Although it is plausible to think that replacing traditional cigarettes with HTPs would lower the risks of COPD, this notion requires confirmation by further investigations from sources independent of the tobacco industry. COPD is characterized by an ongoing inflammatory process in the lungs, and the renin-angiotensin system (RAS) has been implicated in the pathogenesis of COPD. Angiotensin-converting enzyme-2 (ACE2) functions as a negative regulator of RAS and has been suggested as a cellular receptor for the causative agent of SARS-CoV-2. It has been shown that smoking is most likely associated with the negative progression and adverse outcomes of SARS-CoV-2. In this study, we found that cigarette smoke extracts from traditional cigarettes (CSE) caused higher cytotoxicity and higher oxidative stress levels than extracts from HTPs (HTPE) in two lung cell lines (Calu-3 and Beas-2B). CSE and HTPE induced RAS activation, MAPK activation, and NF-kB inflammatory pathway activation, resulting in the production of inflammatory cytokines. Furthermore, CSE and a high dose of HTPE reduced tight junction proteins, including claudin 1, E-cadherin, and ZO-1, and disrupted lung epidermal tight junctions at the air-liquid interface (ALI). Finally, CSE and HTPE enhanced the spike protein S1-induced lung injury response. Together, these results suggest that HTPE induced similar lung pathogenesis relevant to COPD and SARS-CoV-2-induced lung injury caused by CSE.
Keywords: Air-liquid-interface (ALI); Angiotensin-converting enzyme-2 (ACE2); Heated tobacco products; Lung injury; Pulmonary toxicity; SARS-CoV-2; Spike protein.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37.Respir Res. 2019 Nov 9;20(1):251. doi: 10.1186/s12931-019-1226-4. Respir Res. 2019. PMID: 31706310 Free PMC article.
-
Differential Effect of SARS-CoV-2 Spike Glycoprotein 1 on Human Bronchial and Alveolar Lung Mucosa Models: Implications for Pathogenicity.Viruses. 2021 Dec 17;13(12):2537. doi: 10.3390/v13122537. Viruses. 2021. PMID: 34960806 Free PMC article.
-
Lactobacillus rhamnosus Restores Antiviral Signaling and Attenuates Cytokines Secretion from Human Bronchial Epithelial Cells Exposed to Cigarette Smoke and Infected with SARS-CoV-2.Probiotics Antimicrob Proteins. 2023 Dec;15(6):1513-1528. doi: 10.1007/s12602-022-09998-2. Epub 2022 Nov 8. Probiotics Antimicrob Proteins. 2023. PMID: 36346611 Free PMC article.
-
Angiotensin-converting enzyme 2, coronavirus disease 2019, and abdominal aortic aneurysms.J Vasc Surg. 2021 Nov;74(5):1740-1751. doi: 10.1016/j.jvs.2021.01.051. Epub 2021 Feb 15. J Vasc Surg. 2021. PMID: 33600934 Free PMC article. Review.
-
The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice.Exp Physiol. 2008 May;93(5):543-8. doi: 10.1113/expphysiol.2007.040048. Epub 2008 Apr 10. Exp Physiol. 2008. PMID: 18448662 Free PMC article. Review.
Cited by
-
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein S1 Induces Methylglyoxal-Derived Hydroimidazolone/Receptor for Advanced Glycation End Products (MG-H1/RAGE) Activation to Promote Inflammation in Human Bronchial BEAS-2B Cells.Int J Mol Sci. 2023 Oct 3;24(19):14868. doi: 10.3390/ijms241914868. Int J Mol Sci. 2023. PMID: 37834316 Free PMC article.
-
Bronchiectasis-COPD Overlap Syndrome: A Comprehensive Review of its Pathophysiology and Potential Cardiovascular Implications.Ther Adv Pulm Crit Care Med. 2024 Dec 9;19:29768675241300808. doi: 10.1177/29768675241300808. eCollection 2024 Jan-Dec. Ther Adv Pulm Crit Care Med. 2024. PMID: 39655338 Free PMC article. Review.
-
The Product Science of Electrically Heated Tobacco Products: An Updated Narrative Review of the Scientific Literature.Cureus. 2024 May 28;16(5):e61223. doi: 10.7759/cureus.61223. eCollection 2024 May. Cureus. 2024. PMID: 38939262 Free PMC article. Review.
-
Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells.Toxics. 2025 Jan 21;13(2):70. doi: 10.3390/toxics13020070. Toxics. 2025. PMID: 39997888 Free PMC article.
References
-
- Aghapour M., Raee P., Moghaddam S.J., Hiemstra P.S., Heijink I.H. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 2018;58:157–169. - PubMed
-
- Barnes P.J., Burney P.G., Silverman E.K., Celli B.R., Vestbo J., Wedzicha J.A., Wouters E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015;1:15076. - PubMed
-
- Biondi-Zoccai G., Sciarretta S., Bullen C., Nocella C., Violi F., Loffredo L., Pignatelli P., Perri L., Peruzzi M., Marullo A.G.M., De Falco E., Chimenti I., Cammisotto V., Valenti V., Coluzzi F., Cavarretta E., Carrizzo A., Prati F., Carnevale R., Frati G. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking ( SUR - VAPES) 2 Randomized Trial. J. Am. Heart Assoc. 2019;8 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

