BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression
- PMID: 36096532
- PMCID: PMC9472153
- DOI: 10.1136/jitc-2022-004859
BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression
Abstract
Background: Cancer immunotherapy has revolutionized cancer treatment. However, considering the limited success of immunotherapy to only some cancer types and patient cohorts, there is an unmet need for developing new treatments that will result in higher response rates in patients with cancer. Immunoglobulin-like transcript 2 (ILT2), a LILRB family member, is an inhibitory receptor expressed on a variety of immune cells including T cells, natural killer (NK) cells and different myeloid cells. In the tumor microenvironment, binding of class I MHC (in particular HLA-G) to ILT2 on immune cells mediates a strong inhibitory effect, which manifests in inhibition of antitumor cytotoxicity of T and NK cells, and prevention of phagocytosis of the tumor cells by macrophages.
Methods: We describe here the development and characteristics of BND-22, a novel, humanized monoclonal antibody that selectively binds to ILT2 and blocks its interaction with classical MHC I and HLA-G. BND-22 was evaluated for its binding and blocking characteristics as well as its ability to increase the antitumor activity of macrophages, T cells and NK cells in various in vitro, ex vivo and in vivo systems.
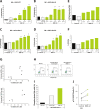
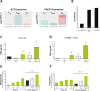
Results: Collectively, our data suggest that BND-22 enhances activity of both innate and adaptive immune cells, thus generating robust and comprehensive antitumor immunity. In humanized mice models, blocking ILT2 with BND-22 decreased the growth of human tumors, hindered metastatic spread to the lungs, and prolonged survival of the tumor-bearing mice. In addition, BND-22 improved the antitumor immune response of approved therapies such as anti-PD-1 or anti-EGFR antibodies.
Conclusions: BND-22 is a first-in-human ILT2 blocking antibody which has demonstrated efficient antitumor activity in various preclinical models as well as a favorable safety profile. Clinical evaluation of BND-22 as a monotherapy or in combination with other therapeutics is under way in patients with cancer.
Trial registration number: NCT04717375.
Keywords: Immunotherapy; Lymphocyte Activation; Macrophages; Translational Medical Research; Tumor Escape.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
