α-Actinin-4 recruits Shp2 into focal adhesions to potentiate ROCK2 activation in podocytes
- PMID: 36096674
- PMCID: PMC9468603
- DOI: 10.26508/lsa.202201557
α-Actinin-4 recruits Shp2 into focal adhesions to potentiate ROCK2 activation in podocytes
Abstract
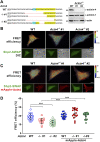
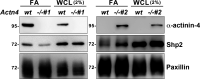
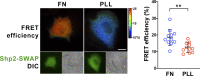
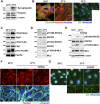
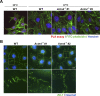
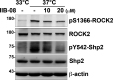
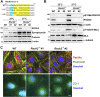
Cell-matrix adhesions are mainly provided by integrin-mediated focal adhesions (FAs). We previously found that Shp2 is essential for FA maturation by promoting ROCK2 activation at FAs. In this study, we further delineated the role of α-actinin-4 in the FA recruitment and activation of Shp2. We used the conditional immortalized mouse podocytes to examine the role of α-actinin-4 in the regulation of Shp2 and ROCK2 signaling. After the induction of podocyte differentiation, Shp2 and ROCK2 were strongly activated, concomitant with the formation of matured FAs, stress fibers, and interdigitating intracellular junctions in a ROCK-dependent manner. Gene knockout of α-actinin-4 abolished the Shp2 activation and subsequently reduced matured FAs in podocytes. We also demonstrated that gene knockout of ROCK2 impaired the generation of contractility and interdigitating intercellular junctions. Our results reveal the role of α-actinin-4 in the recruitment of Shp2 at FAs to potentiate ROCK2 activation for the maintenance of cellular contractility and cytoskeletal architecture in the cultured podocytes.
© 2022 Tseng et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
N-sulfation of heparan sulfate is critical for syndecan-4-mediated podocyte cell-matrix interactions.Am J Physiol Renal Physiol. 2016 May 15;310(10):F1123-35. doi: 10.1152/ajprenal.00603.2015. Epub 2016 Mar 2. Am J Physiol Renal Physiol. 2016. PMID: 26936875 Free PMC article.
-
FSGS-associated alpha-actinin-4 (K256E) impairs cytoskeletal dynamics in podocytes.Kidney Int. 2006 Sep;70(6):1054-61. doi: 10.1038/sj.ki.5001665. Epub 2006 Jul 12. Kidney Int. 2006. PMID: 16837921
-
Shp2 plays a crucial role in cell structural orientation and force polarity in response to matrix rigidity.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2840-5. doi: 10.1073/pnas.1222164110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359696 Free PMC article.
-
Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review.
-
Assembly and mechanosensory function of focal adhesions: experiments and models.Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review.
Cited by
-
Inflammation in glomerular diseases.Front Immunol. 2025 Mar 4;16:1526285. doi: 10.3389/fimmu.2025.1526285. eCollection 2025. Front Immunol. 2025. PMID: 40103820 Free PMC article. Review.
-
Actin Bundles Dynamics and Architecture.Biomolecules. 2023 Feb 28;13(3):450. doi: 10.3390/biom13030450. Biomolecules. 2023. PMID: 36979385 Free PMC article. Review.
-
Targeting Heparanase Attenuates Podocyte Injury Induced by Puromycin Aminonucleoside.J Cell Physiol. 2025 Jun;240(6):e70053. doi: 10.1002/jcp.70053. J Cell Physiol. 2025. PMID: 40495439 Free PMC article.
-
Dynamic regulation of integrin β1 phosphorylation supports invasion of breast cancer cells.Nat Cell Biol. 2025 Jun;27(6):1021-1034. doi: 10.1038/s41556-025-01663-4. Epub 2025 May 26. Nat Cell Biol. 2025. PMID: 40419795 Free PMC article.
-
SHP2: its association and roles in systemic lupus erythematosus.Inflamm Res. 2023 Jul;72(7):1501-1512. doi: 10.1007/s00011-023-01760-w. Epub 2023 Jun 23. Inflamm Res. 2023. PMID: 37351631
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
