Implementation of a provider-focused intervention for maximizing human papillomavirus (HPV) vaccine uptake in young cancer survivors receiving follow-up care in pediatric oncology practices: protocol for a cluster-randomized trial of the HPV PROTECT intervention
- PMID: 36096775
- PMCID: PMC9466329
- DOI: 10.1186/s12887-022-03562-1
Implementation of a provider-focused intervention for maximizing human papillomavirus (HPV) vaccine uptake in young cancer survivors receiving follow-up care in pediatric oncology practices: protocol for a cluster-randomized trial of the HPV PROTECT intervention
Abstract
Background: Childhood cancer survivors are at high risk for developing new cancers (such as cervical and anal cancer) caused by persistent infection with the human papillomavirus (HPV). HPV vaccination is effective in preventing the infections that lead to these cancers, but HPV vaccine uptake is low among young cancer survivors. Lack of a healthcare provider recommendation is the most common reason that cancer survivors fail to initiate the HPV vaccine. Strategies that are most successful in increasing HPV vaccine uptake in the general population focus on enhancing healthcare provider skills to effectively recommend the vaccine, and reducing barriers faced by the young people and their parents in receiving the vaccine. This study will evaluate the effectiveness and implementation of an evidence-based healthcare provider-focused intervention (HPV PROTECT) adapted for use in pediatric oncology clinics, to increase HPV vaccine uptake among cancer survivors 9 to 17 years of age.
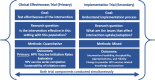
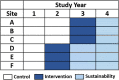
Methods: This study uses a hybrid type 1 effectiveness-implementation approach. We will test the effectiveness of the HPV PROTECT intervention using a stepped-wedge cluster-randomized trial across a multi-state sample of pediatric oncology clinics. We will evaluate implementation (provider perspectives regarding intervention feasibility, acceptability and appropriateness in the pediatric oncology setting, provider fidelity to intervention components and change in provider HPV vaccine-related knowledge and practices [e.g., providing vaccine recommendations, identifying and reducing barriers to vaccination]) using a mixed methods approach.
Discussion: This multisite trial will address important gaps in knowledge relevant to the prevention of HPV-related malignancies in young cancer survivors by testing the effectiveness of an evidence-based provider-directed intervention, adapted for the pediatric oncology setting, to increase HPV vaccine initiation in young cancer survivors receiving care in pediatric oncology clinics, and by procuring information regarding intervention delivery to inform future implementation efforts. If proven effective, HPV PROTECT will be readily disseminable for testing in the larger pediatric oncology community to increase HPV vaccine uptake in cancer survivors, facilitating protection against HPV-related morbidities for this vulnerable population.
Trial registration: ClinicalTrials.gov Identifier: NCT04469569, prospectively registered on July 14, 2020.
Keywords: Childhood cancer survivors; Cluster-randomized trial; Human papillomavirus; Vaccination rates.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Trusted health system implementation strategies to increase vaccination (TRUE SYNERGI): a stepped-wedge cluster randomized trial to reduce HPV-related cancers.BMC Public Health. 2025 Apr 9;25(1):1331. doi: 10.1186/s12889-025-22273-7. BMC Public Health. 2025. PMID: 40205591 Free PMC article. Clinical Trial.
-
Evaluating the impact of multilevel evidence-based implementation strategies to enhance provider recommendation on human papillomavirus vaccination rates among an empaneled primary care patient population: a study protocol for a stepped-wedge cluster randomized trial.Implement Sci. 2018 Jul 13;13(1):96. doi: 10.1186/s13012-018-0778-x. Implement Sci. 2018. PMID: 30001723 Free PMC article. Clinical Trial.
-
Implementation barriers and considerations for recommending and administering the human papillomavirus (HPV) vaccination in oncology settings.J Cancer Surviv. 2024 Oct;18(5):1481-1491. doi: 10.1007/s11764-023-01391-4. Epub 2023 May 6. J Cancer Surviv. 2024. PMID: 37147553 Free PMC article.
-
Human papillomavirus: optimizing opportunities for prevention.Curr Opin Pediatr. 2022 Apr 1;34(2):132-139. doi: 10.1097/MOP.0000000000001119. Curr Opin Pediatr. 2022. PMID: 35152231 Review.
-
Human Papillomavirus Infection and Vaccination.J Pediatr Nurs. 2016 Mar-Apr;31(2):e155-66. doi: 10.1016/j.pedn.2015.10.005. Epub 2015 Nov 14. J Pediatr Nurs. 2016. PMID: 26586310 Review.
Cited by
-
Improving quality and quantity of life for childhood cancer survivors globally in the twenty-first century.Nat Rev Clin Oncol. 2023 Oct;20(10):678-696. doi: 10.1038/s41571-023-00802-w. Epub 2023 Jul 24. Nat Rev Clin Oncol. 2023. PMID: 37488230 Review.
-
Pediatric Oncology Providers' HPV Vaccine Knowledge, Attitude, Self-Efficacy, and Practice after Communication Training: A Comparison with a National Survey.Vaccines (Basel). 2024 Sep 17;12(9):1060. doi: 10.3390/vaccines12091060. Vaccines (Basel). 2024. PMID: 39340090 Free PMC article.
-
A framework-based guide for adapting and implementing primary care-based pediatric interventions to the pediatric oncology setting: HPV PROTECT as an exemplar.Cancer. 2025 May 1;131(9):e35857. doi: 10.1002/cncr.35857. Cancer. 2025. PMID: 40297935 Free PMC article.
-
Examining the Barriers and Opportunities for Human Papillomavirus Vaccine Delivery in Cancer Care Settings: A Mixed-Methods Study.Cancer Prev Res (Phila). 2023 Oct 2;16(10):581-589. doi: 10.1158/1940-6207.CAPR-23-0046. Cancer Prev Res (Phila). 2023. PMID: 37258419 Free PMC article.
-
Human Papillomavirus Vaccination in Pediatric, Adolescent, and Young Adult Cancer Survivors-Opportunity to Address Gaps in Cancer Prevention and Survivorship.Vaccines (Basel). 2024 Jan 24;12(2):114. doi: 10.3390/vaccines12020114. Vaccines (Basel). 2024. PMID: 38400098 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical