Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss
- PMID: 36096817
- PMCID: PMC9465898
- DOI: 10.1186/s12974-022-02585-w
Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss
Abstract
Background: Chronic suppurative otitis media (CSOM) is the most common cause of permanent hearing loss in children in the developing world. A large component of the permanent hearing loss is sensory in nature and our understanding of the mechanism of this has so far been limited to post-mortem human specimens or acute infection models that are not representative of human CSOM. In this report, we assess cochlear injury in a validated Pseudomonas aeruginosa (PA) CSOM mouse model.
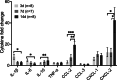
Methods: We generated persisters (PCs) and inoculated them into the mouse middle ear cavity. We tracked infection with IVIS and detected PA using RT-PCR. We assessed cochlear damage and innate immunity by Immunohistochemistry. Finally, we evaluated cytokines with multiplex assay and quantitative real-time PCR.
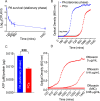
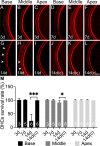
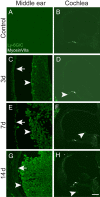
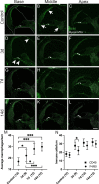
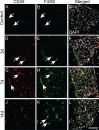
Results: We observed outer hair cell (OHC) loss predominantly in the basal turn of the cochlear at 14 days after bacterial inoculation. Macrophages, not neutrophils are the major immune cells in the cochlea in CSOM displaying increased numbers and a distribution correlated with the observed cochlear injury. The progression of the morphological changes suggests a transition from monocytes into tissue macrophages following infection. We also show that PA do not enter the cochlea and live bacteria are required for cochlear injury. We characterized cytokine activity in the CSOM cochlea.
Conclusions: Taken together, this data shows a critical role for macrophages in CSOM-mediated sensorineural hearing loss (SNHL).
Keywords: CSOM; Cytokines; HC loss; Macrophages; PA; SNHL.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures

References
-
- Santa Maria PL, Kaufman AC, Bacacao B, Thai A, Chen X, Xia A, et al. Topical Therapy Failure in Chronic Suppurative Otitis Media is Due to Persister Cells in Biofilms. Otol Neurotol. 2021. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

