Effectiveness of a complex regional advance care planning intervention to improve care consistency with care preferences: study protocol for a multi-center, cluster-randomized controlled trial focusing on nursing home residents (BEVOR trial)
- PMID: 36096948
- PMCID: PMC9465132
- DOI: 10.1186/s13063-022-06576-3
Effectiveness of a complex regional advance care planning intervention to improve care consistency with care preferences: study protocol for a multi-center, cluster-randomized controlled trial focusing on nursing home residents (BEVOR trial)
Abstract
Background: According to recent legislation, facilitated advance care planning (ACP) for nursing home (NH) residents is covered by German sickness funds. However, the effects of ACP on patient-relevant outcomes have not been studied in Germany yet. This study investigates whether implementing a complex regional ACP intervention improves care consistency with care preferences in NH residents.
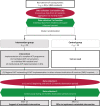
Methods: This is a parallel-group cluster-randomized controlled trial (cRCT) with 48 NHs (≈ 3840 resident beds) between 09/2019 and 02/2023. The intervention group will receive a complex, regional ACP intervention aiming at sustainable systems redesign at all levels (individual, institutional, regional). The intervention comprises comprehensive training of ACP facilitators, implementation of reliable ACP processes, organizational development in the NH and other relevant institutions of the regional healthcare system, and education of health professionals caring for the residents. Control group NHs will deliver care as usual. Primary outcome is the hospitalization rate during the 12-months observation period. Secondary outcomes include the rate of residents whose preferences were known and honored in potentially life-threatening events, hospital days, index treatments like resuscitation and artificial ventilation, advance directives, quality of life, psychological burden on bereaved families, and costs of care. The NHs will provide anonymous, aggregated data of all their residents on the primary outcome and several secondary outcomes (data collection 1). For residents who have given informed consent, we will evaluate care consistency with care preferences and further secondary outcomes, based on chart reviews and short interviews with residents, surrogates, and carers (data collection 2). Process evaluation will aim to explain barriers and facilitators, economic evaluation the cost implications.
Discussion: This study has the potential for high-quality evidence on the effects of a complex regional ACP intervention on NH residents, their families and surrogates, NH staff, and health care utilization in Germany. It is the first cRCT investigating a comprehensive regional ACP intervention that aims at improving patient-relevant clinical outcomes, addressing and educating multiple institutions and health care providers, besides qualification of ACP facilitators. Thereby, it can generate evidence on the potential of ACP to effectively promote patient-centered care in the vulnerable population of frail and often chronically ill elderly.
Trial registration: ClinicalTrials.gov ID NCT04333303 . Registered 30 March 2020.
Keywords: ACP facilitation; Advance care planning; Cluster-randomized controlled trial; Complex intervention; Nursing homes; Patient-centered care; Study protocol.
© 2022. The Author(s).
Conflict of interest statement
Berend Feddersen, Angela Fuchs, Kornelia Götze, Georg Marckmann, Jürgen in der Schmitten, Henrikje Stanze, Friedemann Nauck, and Jan Schildmann are ACP trainers and received professional fees for conducting ACP qualifications, talks, or workshops on ACP outside of the study. Jürgen in der Schmitten (2011-2021) has been and Kornelia Götze is (since 08/2021) treasurer of the international ACP-i society. Jürgen in der Schmitten (since 02/2017), Friedemann Nauck (02/2017-06/2021), Berend Feddersen (06/2019-06/2021), Kornelia Götze (since 06/2021), and Georg Marckmann (since 02/2017) were or are committee members of the Advance Care Planning Germany society.
Figures


References
-
- Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–ee51. doi: 10.1016/S1470-2045(17)30582-X. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

