Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial
- PMID: 36097029
- PMCID: PMC9467419
- DOI: 10.1038/s41467-022-33036-y
Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial
Abstract
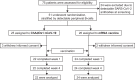
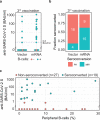
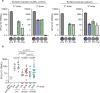
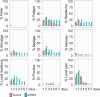
Impaired response to COVID-19 vaccination is of particular concern in immunosuppressed patients. To determine the best vaccination strategy for this vulnerable group we performed a single center, 1:1 randomized blinded clinical trial. Patients who failed to seroconvert upon two mRNA vaccinations (BNT162b2 or mRNA-1273) are randomized to receive either a third dose of the same mRNA or the vector vaccine ChAdOx1 nCoV-19. Primary endpoint is the difference in SARS-CoV-2 spike antibody seroconversion rate between vector and mRNA vaccinated patients four weeks after the third dose. Secondary outcomes include cellular immune responses. Seroconversion rates at week four are significantly higher in the mRNA (homologous vaccination, 15/24, 63%) as compared to the vector vaccine group (heterologous vaccination, 4/22, 18%). SARS-CoV-2-specific T-cell responses are reduced but could be increased after a third dose of either vector or mRNA vaccine. In a multivariable logistic regression analysis, patient age and vaccine type are associated with seroconversion. No serious adverse event is attributed to COVID-19 booster vaccination. Efficacy and safety data underline the importance of a booster vaccination and support the use of a homologous mRNA booster vaccination in immunosuppressed patients.Trial registration: EudraCT No.: 2021-002693-10.
© 2022. The Author(s).
Conflict of interest statement
P.M. reports speaker fees from AbbVie, Janssen, and Novartis and research grants from AbbVie, BMS, Novartis, Janssen, MSD, and UCB. M.B. reports about personal fees from Eli Lilly, D.A. received grants and consulting fees from AbbVie, Amgen, Lilly, Merck, Novartis, Pfizer, Roche, and Sandoz, J.S. reports about grants, consulting, and personal fees from AbbVie, Astra-Zeneca, Lilly, Novartis, Amgen, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO, Janssen, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, Samsung, and UCB, M.Z. received grants and consulting fees from Nabriva, AntibioTxApS, Shionogi, NovoNordisk, Merck, Infectopharm, and Pfizer, H.H. received grants from Glock Health, BlueSky Immunotherapies, and Neutrolis. G.N. served as a speaker/consultant/advisory board member for AbbVie, MSD, Takeda, Janssen, Sandoz, Pfizer, Astro Pharma, Falk Pharma GmbH, Ferring, Gilead, Galapagos, and Vifor. E.S. reports support for meeting attendances from Pfizer and Bristol Myers Squibb. D.M. received support for meeting attendances from Pfizer. T.R. received grant support from Abbvie, Boehringer-Ingelheim, Gilead, Gore, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, and Siemens; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Abbvie, Boehringer-Ingelheim, Gilead, and Roche. A.K. reports about speaker and consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Gilead, Janssen, Merck Sharp and Dohme, Novartis, and Pfizer. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

