Lassa fever - the road ahead
- PMID: 36097163
- PMCID: PMC9466315
- DOI: 10.1038/s41579-022-00789-8
Lassa fever - the road ahead
Abstract
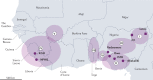
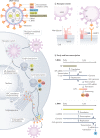
Lassa virus (LASV) is endemic in the rodent populations of Sierra Leone, Nigeria and other countries in West Africa. Spillover to humans occurs frequently and results in Lassa fever, a viral haemorrhagic fever (VHF) associated with a high case fatality rate. Despite advances, fundamental gaps in knowledge of the immunology, epidemiology, ecology and pathogenesis of Lassa fever persist. More frequent outbreaks, the potential for further geographic expansion of Mastomys natalensis and other rodent reservoirs, the ease of procurement and possible use and weaponization of LASV, the frequent importation of LASV to North America and Europe, and the emergence of novel LASV strains in densely populated West Africa have driven new initiatives to develop countermeasures for LASV. Although promising candidates are being evaluated, as yet there are no approved vaccines or therapeutics for human use. This Review discusses the virology of LASV, the clinical course of Lassa fever and the progress towards developing medical countermeasures.
© 2022. Springer Nature Limited.
Conflict of interest statement
R.F.G. is the co-founder of Zalgen Labs, a biotechnology company developing countermeasures for Lassa virus (LASV) and other emerging viruses.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

