Comparison of topical benzyl benzoate vs. oral ivermectin in treating scabies: A randomized study
- PMID: 36097258
- PMCID: PMC10087012
- DOI: 10.1111/jdv.18573
Comparison of topical benzyl benzoate vs. oral ivermectin in treating scabies: A randomized study
Abstract
Background: Scabies is an itchy, parasitic infection of the skin. Recent reports indicate there is a decreasing efficacy of the standard treatment of choice, topical 5% permethrin cream.
Objective: To evaluate the comparative efficacy, safety and tolerability of topical benzyl benzoate (BB) with oral ivermectin in the treatment of scabies.
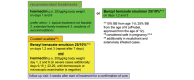
Methods: Patients with dermoscopy-verified scabies visiting the dermatologic outpatient clinic were assessed for enrolment in the study. In total, 224 patients were enrolled and sequentially randomized into two equally sized groups. Group A received topical 25% or 10% BB for the daily use over a period of three consecutive days, group B received oral ivermectin (200 μg/kg body weight) twice, 1 week apart. Treatment outcome was evaluated by dermoscopy at a 3-week follow-up visit.
Results: Treatment resulted in a cure rate of 87% in group A and 86% in group B. After initial therapy failure in group A, six out of eight patients showed treatment response upon repeated application of BB, five of five when retreated with ivermectin and two of two with BB plus ivermectin, respectively. In group B, successful retreatment was observed in three out of three patients with ivermectin, two of two patients with BB and 11 of 11 patients with the combination of BB plus ivermectin, respectively. Tolerability and safety profile of oral ivermectin was excellent, while BB produced short burning sensations in 14%.
Conclusion: Topical BB and oral ivermectin have shown comparable good therapeutic efficacy. Therefore, both agents constitute an adequate first-line therapy in the treatment of scabies. A combination of both agents may be considered in recalcitrant and extensively infested cases, additionally to crusted scabies.
© 2022 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
None declared.
Figures
References
-
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248–53. - PubMed
-
- Sunderkötter C, Feldmeier H, Fölster‐Holst R, Geisel B, Klinke‐Rehbein S, Nast A, et al. S1 guidelines on the diagnosis and treatment of scabies – short version. J Dtsch Dermatol Ges. 2016;14:1155–67. Extended version at: www.awmf.org - PubMed
-
- Österreichische Gesellschaft für Sexually Transmitted Diseases und dermatologische Mikrobiologie: Skabies Therapiemanagement für Allgemeinmediziner und Fachärzte. Addendum zur Version 11/2019. Available from: http://www.oegstd.at/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

