Multimodal prediction of residual consciousness in the intensive care unit: the CONNECT-ME study
- PMID: 36097353
- PMCID: PMC9825454
- DOI: 10.1093/brain/awac335
Multimodal prediction of residual consciousness in the intensive care unit: the CONNECT-ME study
Abstract
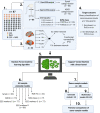
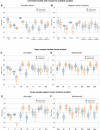
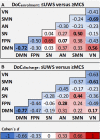
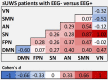
Functional MRI (fMRI) and EEG may reveal residual consciousness in patients with disorders of consciousness (DoC), as reflected by a rapidly expanding literature on chronic DoC. However, acute DoC is rarely investigated, although identifying residual consciousness is key to clinical decision-making in the intensive care unit (ICU). Therefore, the objective of the prospective, observational, tertiary centre cohort, diagnostic phase IIb study 'Consciousness in neurocritical care cohort study using EEG and fMRI' (CONNECT-ME, NCT02644265) was to assess the accuracy of fMRI and EEG to identify residual consciousness in acute DoC in the ICU. Between April 2016 and November 2020, 87 acute DoC patients with traumatic or non-traumatic brain injury were examined with repeated clinical assessments, fMRI and EEG. Resting-state EEG and EEG with external stimulations were evaluated by visual analysis, spectral band analysis and a Support Vector Machine (SVM) consciousness classifier. In addition, within- and between-network resting-state connectivity for canonical resting-state fMRI networks was assessed. Next, we used EEG and fMRI data at study enrolment in two different machine-learning algorithms (Random Forest and SVM with a linear kernel) to distinguish patients in a minimally conscious state or better (≥MCS) from those in coma or unresponsive wakefulness state (≤UWS) at time of study enrolment and at ICU discharge (or before death). Prediction performances were assessed with area under the curve (AUC). Of 87 DoC patients (mean age, 50.0 ± 18 years, 43% female), 51 (59%) were ≤UWS and 36 (41%) were ≥ MCS at study enrolment. Thirty-one (36%) patients died in the ICU, including 28 who had life-sustaining therapy withdrawn. EEG and fMRI predicted consciousness levels at study enrolment and ICU discharge, with maximum AUCs of 0.79 (95% CI 0.77-0.80) and 0.71 (95% CI 0.77-0.80), respectively. Models based on combined EEG and fMRI features predicted consciousness levels at study enrolment and ICU discharge with maximum AUCs of 0.78 (95% CI 0.71-0.86) and 0.83 (95% CI 0.75-0.89), respectively, with improved positive predictive value and sensitivity. Overall, both machine-learning algorithms (SVM and Random Forest) performed equally well. In conclusion, we suggest that acute DoC prediction models in the ICU be based on a combination of fMRI and EEG features, regardless of the machine-learning algorithm used.
Keywords: EEG; acute brain injury; disorders of consciousness; functional MRI; machine-learning.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

Comment in
-
Beyond crystal balls: multimodal prediction of early recovery of consciousness.Brain. 2023 Jan 5;146(1):6-7. doi: 10.1093/brain/awac434. Brain. 2023. PMID: 36508392 No abstract available.
References
-
- Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health. 2016;1:e76–e83. - PubMed
-
- Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098. - PubMed
-
- Kondziella D, Frontera JA. Pearls & Oy-sters: eyes-open coma. Neurology. 2021;96:864–867. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

