Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications
- PMID: 36097597
- PMCID: PMC9463390
- DOI: 10.1016/j.mtbio.2022.100412
Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications
Abstract
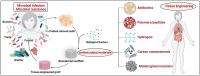
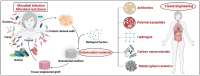
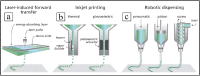
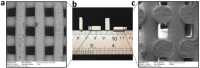
Due to microbial infections dramatically affect cell survival and increase the risk of implant failure, scaffolds produced with antimicrobial materials are now much more likely to be successful. Multidrug-resistant infections without suitable prevention strategies are increasing at an alarming rate. The ability of cells to organize, develop, differentiate, produce a functioning extracellular matrix (ECM) and create new functional tissue can all be controlled by careful control of the extracellular microenvironment. This review covers the present state of advanced strategies to develop scaffolds with antimicrobial properties for bone, oral tissue, skin, muscle, nerve, trachea, cardiac and other tissue engineering applications. The review focuses on the development of antimicrobial scaffolds against bacteria and fungi using a wide range of materials, including polymers, biopolymers, glass, ceramics and antimicrobials agents such as antibiotics, antiseptics, antimicrobial polymers, peptides, metals, carbon nanomaterials, combinatorial strategies, and includes discussions on the antimicrobial mechanisms involved in these antimicrobial approaches. The toxicological aspects of these advanced scaffolds are also analyzed to ensure future technological transfer to clinics. The main antimicrobial methods of characterizing scaffolds' antimicrobial and antibiofilm properties are described. The production methods of these porous supports, such as electrospinning, phase separation, gas foaming, the porogen method, polymerization in solution, fiber mesh coating, self-assembly, membrane lamination, freeze drying, 3D printing and bioprinting, among others, are also included in this article. These important advances in antimicrobial materials-based scaffolds for regenerative medicine offer many new promising avenues to the material design and tissue-engineering communities.
Keywords: Antimicrobial activity; Biomaterials; Fabrication; Scaffolds; Tissue engineering.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Ambekar R.S., Kandasubramanian B. Progress in the advancement of porous biopolymer scaffold: tissue engineering application. Ind. Eng. Chem. Res. 2019;58:6163–6194. doi: 10.1021/acs.iecr.8b05334. - DOI
-
- DE Boer J.B. third ed. Academic Press; Oxford, United Kingdom: 2022. TISSUE ENGINEERING.
-
- Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E. Academic Press; Toronto, Canada: 2012. Biomaterials Science: an Introduction to Materials in Medicine.
-
- Moreno-Manzano V., Zaytseva-Zotova D., López-Mocholí E., Briz-Redón Á., Strand B.L., Serrano-Aroca Á. Injectable gel form of a decellularized bladder induces adipose-derived stem cell differentiation into smooth muscle cells in vitro. Int. J. Mol. Sci. 2020;21 doi: 10.3390/IJMS21228608. 8608. 21 (2020) 8608. - DOI - PMC - PubMed

