Effect of endometrial cell-conditioned medium and platelet-rich plasma on the developmental competence of mouse preantral follicles: An in vitro study
- PMID: 36097733
- PMCID: PMC9468696
- DOI: 10.5653/cerm.2022.05260
Effect of endometrial cell-conditioned medium and platelet-rich plasma on the developmental competence of mouse preantral follicles: An in vitro study
Abstract
Objective: The aim of this study was to evaluate the impacts of platelet-rich plasma (PRP) and conditioned medium (CM) derived from endometrial stromal cells on mouse preantral follicle culture in a two-dimensional system to produce competent mature oocytes for fertilization.
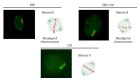
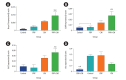
Methods: In total, 240 preantral follicles were isolated from female mouse ovarian tissue and divided into four groups. The preantral follicles were isolated three times for each group and then cultured, respectively, in the presence of alpha minimum essential medium (control), PRP, CM, and PRP+CM. The in vitro growth, in vitro maturation, and cleavage percentage of the preantral follicles were investigated. Immunocytochemistry (IHC) was also conducted to monitor the meiotic progression of the oocytes. Additionally, the mRNA expression levels of the two folliculogenesis-related genes (Gdf9 and Bmp15) and two apoptosis-related genes (Bcl2 and Bax) were investigated using real-time polymerase chain reaction.
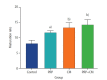
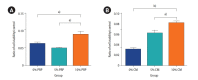
Results: In the PRP, CM, and PRP+CM groups, the preantral follicle maturation (evaluated by identifying polar bodies) were greater than the control group. The cleavage rate in the CM, and PRP+CM groups were also greater than the control group. IHC analysis demonstrated that in each treatment group, meiotic spindle was normal. In the PRP+CM group, the gene expression levels of Bmp15, Gdf9, and Bcl2 were greater than in the other groups. The Bax gene was more strongly expressed in the PRP and control groups than in the other groups.
Conclusion: Overall, the present study suggests that the combination of CM and PRP can effectively increase the growth and cleavage rate of mouse preantral follicles in vitro.
Keywords: Conditioned medium; Culture; Endometrial cells; Platelet-rich plasma; Preantral follicles.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Motamed M, Sadr Z, Valojerdi MR, Moini A, Oryan S, Totonchi M, et al. Tissue engineered human amniotic membrane application in mouse ovarian follicular culture. Ann Biomed Eng. 2017;45:1664–75. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

