Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: The multicenter prospective cohort GARLIT study
- PMID: 36097739
- PMCID: PMC10086852
- DOI: 10.1111/ene.15563
Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: The multicenter prospective cohort GARLIT study
Abstract
Background and purpose: To evaluate the 1-year effectiveness and tolerability of galcanezumab in real life and the prognostic indicators of persistent response.
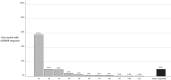
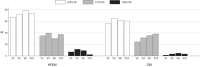
Methods: High-frequency episodic migraine (HFEM) and chronic migraine (CM) patients treated with galcanezumab who completed a 1-year observation were enrolled. The primary outcomes assessed during the 12 months (V1-V12) were the change in monthly migraine days (MMDs) from baseline and the response rates ≥50% in MMDs (MMD ≥50% RR). The secondary outcomes were changes in pain intensity (numerical rating scale [NRS]) and in monthly acute medication intake (MAMI).
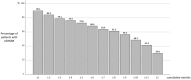
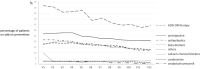
Results: We enrolled 191 patients (77.5% CM). Twenty-three patients (12%) dropped out, two for nonserious adverse events. At least 40% of patients took add-on standard preventives from baseline to V12. At V12, MMDs were reduced by 6.0 days in HFEM and by 11.9 days in CM patients (both p < 0.00001); NRS and MAMI were also decreased in both groups (p < 0.00001). One-hundred eight (56.5%) patients presented MMD ≥50% RR for 9 cumulative months (interquartile range=8): we defined this value as the cutoff for a persistent response. Persistent responders were less likely to have a higher body mass index (BMI) (p = 0.007) but more frequently had a good response to triptans (p = 0.005) and MMD ≥50% RR at V1 (p < 0.0000001). Patients without a persistent response were on add-on therapy for longer periods of time (p < 0.001).
Conclusions: Galcanezumab was effective and well-tolerated in the 1-year term, with most patients presenting MMD ≥50% RR for at least 9 months. Triptan response, lower BMI, and MMD ≥50% RR in the first month emerged as predictive factors for a persistent response.
Trial registration: ClinicalTrials.gov NCT0480351.
Keywords: CGRP; galcanezumab; migraine; monoclonal antibodies; real life.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
M.Ag. received grants from Novartis and Lilly. M.Al. received honoraria or travel grants from Novartis, Teva, Merck Serono, Almirall, and Biogen. C.Al. received grants and honoraria from Lusofarmaco, Laborest, Abbvie, Novartis, and Eli Lilly. C.Au. received travel grants and honoraria from FB‐Health, Lusofarmaco, Almirall, Eli‐Lilly, Novartis, and Teva. P.B. received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Alder, Allergan, Angelini, Assosalute, Bayer, ElectroCore, Eli‐Lilly, GSK, Lundbeck, Lusofarmaco, 1MED, MSD, New Penta, Noema Pharma, Novartis, Stx‐Med, Teva, Visufarma, and Zambon. F.B. received honoraria as a speaker or for participating in advisory boards from Teva, Novartis, and Ipsen. S.C. received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Lilly, Allergan, Ibsa, Amgen, and Lundbeck. V.D.P. received grants and honoraria by Bayer, Biogen, Lilly, TEVA, and Novartis. G.E. received travel grants and honoraria from Eli‐Lilly, Novartis, New Penta, and Ecupharma. V.F. has received honoraria as speaker or for participating in advisory boards from Ely‐Lilly. C.F. received travel grants, honoraria Lilly, TEVA, and Aim Group. C.L. received grants from Novartis and Lilly. I.M. received honoraria from Eli Lilly. A.P. received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Eli‐Lilly, Novartis, and Teva. A.R. received speaker honoraria from Teva and Lilly. R.R. received honoraria for speaker panels from Teva, Lilly, and Novartis. F.V. received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan‐Abbvie, Amgen, Angelini, Eli‐Lilly, Lundbeck, Novartis, and Teva. N.B., L.d.O., and M.M. have no competing interest.
Figures

Similar articles
-
Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study).J Headache Pain. 2021 May 3;22(1):35. doi: 10.1186/s10194-021-01247-1. J Headache Pain. 2021. PMID: 33941080 Free PMC article.
-
Conversion from chronic to episodic migraine in patients treated with galcanezumab in real life in Italy: the 12-month observational, longitudinal, cohort multicenter GARLIT experience.J Neurol. 2022 Nov;269(11):5848-5857. doi: 10.1007/s00415-022-11226-4. Epub 2022 Jun 28. J Neurol. 2022. PMID: 35763113 Free PMC article.
-
Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study.J Headache Pain. 2021 Dec 18;22(1):154. doi: 10.1186/s10194-021-01363-y. J Headache Pain. 2021. PMID: 34922444 Free PMC article.
-
Efficacy and Safety of Galcanezumab for the Preventive Treatment of Migraine: A Narrative Review.Adv Ther. 2020 May;37(5):2034-2049. doi: 10.1007/s12325-020-01319-9. Epub 2020 Apr 21. Adv Ther. 2020. PMID: 32319039 Free PMC article. Review.
-
Different doses of galcanezumab versus placebo in patients with migraine and cluster headache: a meta-analysis of randomized controlled trials.J Headache Pain. 2020 Feb 11;21(1):14. doi: 10.1186/s10194-020-1085-x. J Headache Pain. 2020. PMID: 32046655 Free PMC article.
Cited by
-
Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data.Cells. 2022 Dec 29;12(1):143. doi: 10.3390/cells12010143. Cells. 2022. PMID: 36611935 Free PMC article.
-
Nine-Month Continuous Fremanezumab Prophylaxis on the Response to Triptans and Also on the Incidence of Triggers, Hypersensitivity and Prodromal Symptoms of Patients with High-Frequency Episodic Migraine.J Clin Med. 2024 Jan 10;13(2):386. doi: 10.3390/jcm13020386. J Clin Med. 2024. PMID: 38256516 Free PMC article.
-
Changes in Health-Related Quality of Life in Patients with Therapy-Resistant Migraine during Treatment with Erenumab in an Ambulatory Care Setting.J Clin Med. 2023 Aug 28;12(17):5619. doi: 10.3390/jcm12175619. J Clin Med. 2023. PMID: 37685685 Free PMC article.
-
Fremanezumab for Episodic Migraine Prevention in Japanese Patients: Subgroup Analysis from Two International Trials.J Pain Res. 2023 May 18;16:1673-1682. doi: 10.2147/JPR.S393896. eCollection 2023. J Pain Res. 2023. PMID: 37223438 Free PMC article.
-
Long-Term Effectiveness of Galcanezumab in the Prevention of Migraine: An Italian Retrospective Analysis (REALITY).Neurol Ther. 2024 Apr;13(2):415-435. doi: 10.1007/s40120-024-00582-0. Epub 2024 Feb 8. Neurol Ther. 2024. PMID: 38329615 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

