Neuroprotection of Low-Frequency Repetitive Transcranial Magnetic Stimulation after Ischemic Stroke in Rats
- PMID: 36097798
- PMCID: PMC10042643
- DOI: 10.1002/ana.26509
Neuroprotection of Low-Frequency Repetitive Transcranial Magnetic Stimulation after Ischemic Stroke in Rats
Abstract
Objective: Stroke is a leading cause of human death and disability. Effective early treatments with reasonable therapeutic windows remain critically important to improve the outcomes of stroke. Transcranial magnetic stimulation (TMS) is an established noninvasive technique that has been applied clinically and in animal research for multiple brain disorders, but few studies have examined acute neuroprotection against ischemic stroke. The present investigation tested the novel approach of low-frequency repetitive TMS (rTMS) as an acute treatment after ischemic stroke.
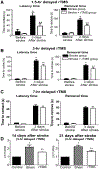
Methods: Adult male rats received focal ischemic surgery through occlusion of the right middle cerebral artery for 60 minutes. The rats received either rTMS or sham treatment with 1.5-, 3-, 4-, or 7-hour delay after the onset of stroke. Low-frequency and low-intensity rTMS was applied to the rat brain for two 30-minute episodes separated by a 1-hour interval.
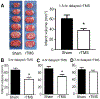
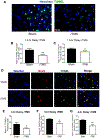
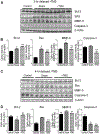
Results: Three days after stroke, compared to stroke controls, rats receiving rTMS treatment with a 1.5-hour delay showed a 35% reduction of infarct volume. Protective effects were also seen with 3- or 4-hour-delayed treatments by rTMS, shown as reduced infarct volume and cell death. rTMS treatment upregulated the antiapoptotic factor Bcl-2 and downregulated the proapoptotic caspase-3 cleavage, expressions of Bax and matrix metallopeptidase-9. In sensorimotor functional assessments 3 to 21 days after stroke, rats receiving rTMS treatment with a 1.5- or 3-hour delay showed significantly better performance compared to stroke controls.
Interpretation: These results support the inference that low-frequency rTMS may be feasible as a neuroprotective acute treatment after ischemic stroke. ANN NEUROL 2023;93:336-347.
© 2022 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
Nothing to report.
Figures

References
-
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

