A prospective study on myocardial injury after BNT162b2 mRNA COVID-19 fourth dose vaccination in healthy persons
- PMID: 36097844
- PMCID: PMC9538001
- DOI: 10.1002/ejhf.2687
A prospective study on myocardial injury after BNT162b2 mRNA COVID-19 fourth dose vaccination in healthy persons
Abstract
Aims: To prospectively evaluate the incidence of myocardial injury after the administration of the fourth dose BNT162b2 mRNA vaccine (Pfizer-BioNTech) against COVID-19.
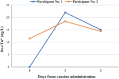
Methods and results: Health care workers who received the BNT162b2 vaccine during the fourth dose campaign had blood samples collected for high-sensitivity cardiac troponin (hs-cTn) during vaccine administration and 2-4 days afterward. Vaccine-related myocardial injury was defined as hs-cTn elevation above the 99th percentile upper reference limit and >50% increase from baseline measurement. Participants with evidence of myocardial injury underwent assessment for possible myocarditis. Of 324 participants, 192 (59.2%) were female and the mean age was 51.8 ± 15.0 years. Twenty-one (6.5%) participants had prior COVID-19 infection, the mean number of prior vaccine doses was 2.9 ± 0.4, and the median time from the last dose was 147 (142-157) days. Reported vaccine-related adverse reactions included local pain at injection site in 57 (17.59%), fatigue in 39 (12.04%), myalgia in 32 (9.88%), sore throat in 21 (6.48%), headache in 18 (5.5%), fever ≥38°C in 16 (4.94%), chest pain in 12 (3.7%), palpitations in 7 (2.16%), and shortness of breath in one (0.3%) participant. Vaccine-related myocardial injury was demonstrated in two (0.62%) participants, one had mild symptoms and one was asymptomatic; both had a normal electrocardiogram and echocardiography.
Conclusion: In a prospective investigation, an increase in serum troponin levels was documented among 0.62% of healthy health care workers receiving the fourth dose BNT162b2 vaccine. The two cases had mild or no symptoms and no clinical sequela.
Clinical trial registration: ClinicalTrials.gov Identifier: NCT05308680.
Keywords: COVID-19; Myocardial injury; Myocarditis; Vaccine; mRNA.
© 2022 European Society of Cardiology.
Figures
References
-
- Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer‐BioNTech COVID‐19 vaccination. Pediatrics. 2021;148:e2021052478. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical