Extracellular Vesicles Regulate Sympatho-Excitation by Nrf2 in Heart Failure
- PMID: 36098045
- PMCID: PMC9529870
- DOI: 10.1161/CIRCRESAHA.122.320916
Extracellular Vesicles Regulate Sympatho-Excitation by Nrf2 in Heart Failure
Abstract
Background: Chronic heart failure (CHF) is associated with redox imbalance. Downregulation of Nrf2 (nuclear factor [erythroid-derived 2]-like 2) plays important roles in disrupting myocardial redox homeostasis and mediating sympathetic nerve activity in the setting of CHF. However, it is unclear if circulating extracellular vesicles (EVs) elicit sympathetic excitation in CHF by disrupting central redox homeostasis. We tested the hypothesis that cardiac-derived EVs circulate to the presympathetic rostral ventrolateral medulla and contribute to oxidative stress and sympathetic excitation via EV-enriched microRNA-mediated Nrf2 downregulation.
Methods: Data were collected on rats with CHF post-myocardial infarction (MI) and on human subjects with ischemic CHF. EVs were isolated from tissue and plasma, and we determined the miRNAs cargo that related to targeting Nrf2 translation. We tracked the distribution of cardiac-derived EVs using in vitro labeled circulating EVs and cardiac-specific membrane GFP+ transgenic mice. Finally, we tested the impact of exogenously loading of antagomirs to specific Nrf2-related miRNAs on CHF-EV-induced pathophysiological phenotypes in normal rats (eg, sympathetic and cardiac function).
Results: Nrf2 downregulation in CHF rats was associated with an upregulation of Nrf2-targeting miRNAs, which were abundant in cardiac-derived and circulating EVs from rats and humans. EVs isolated from the brain of CHF rats were also enriched with Nrf2-targeting miRNAs and cardiac-specific miRNAs. Cardiac-derived EVs were taken up by neurons in the rostral ventrolateral medulla. The administration of cardiac-derived and circulating EVs from CHF rats into the rostral ventrolateral medulla of normal rats evoked an increase in renal sympathetic nerve activity and plasma norepinephrine compared with Sham-operated rats, which were attenuated by exogenously preloading CHF-EVs with antagomirs to Nrf2-targeting miRNAs.
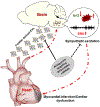
Conclusions: Cardiac microRNA-enriched EVs from animals with CHF can mediate crosstalk between the heart and the brain in the regulation of sympathetic outflow by targeting the Nrf2/antioxidant signaling pathway. This new endocrine signaling pathway regulating sympathetic outflow in CHF may be exploited for novel therapeutics.
Keywords: antioxidant; extracellular vesicles; heart failure; oxidative stress; sympathetic nervous system.
Conflict of interest statement
DISCLOSURES
The authors declare no competing interests.
Figures
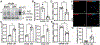






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

