Towards Substrate-Reagent Interaction of Lochmann-Schlosser Bases in THF: Bridging THF Hides Potential Reaction Site of a Chiral Superbase
- PMID: 36098179
- PMCID: PMC10092790
- DOI: 10.1002/chem.202202660
Towards Substrate-Reagent Interaction of Lochmann-Schlosser Bases in THF: Bridging THF Hides Potential Reaction Site of a Chiral Superbase
Abstract
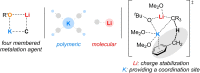
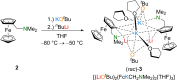
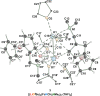
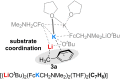
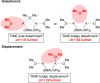
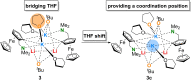
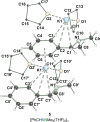
The metalation of N,N-dimethylaminomethylferrocene in THF by the superbasic mixture of n BuLi/KOt Bu proceeds readily at low temperatures to afford a bimetallic Li2 K2 aggregate containing ferrocenyl anions and tert-butoxide. Starting from an enantiomerically enriched ortho-lithiated aminomethylferrocene, an enantiomerically pure superbase can be prepared. The molecular compound exhibits superbasic behavior deprotonating N,N-dimethylbenzylamine in the α-position and is also capable of deprotonating toluene. Quantum chemical calculations provide insight into the role of the bridging THF molecule to the possible substrate-reagent interaction. In addition, a benzylpotassium alkoxide adduct gives a closer look into the corresponding reaction site of the Lochmann-Schlosser base that is reported herein.
Keywords: X-ray diffraction; alkali metals; chirality; lithium; potassium.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

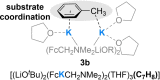

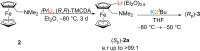

References
-
- None
-
- Lochmann L., Pospĩsil J., Lím D., Tetrahedron Lett. 1966, 7, 257–262;
-
- Schlosser M., J. Organomet. Chem. 1967, 8, 9–16;
-
- Lochmann L., Janata M., Cent. Eur. J. Chem. 2014, 12 537–548; For a review on Alkali-Metal Mediated Synergistic Effects in Polar Main Group Organometallic Chemistry: - PubMed
-
- Robertson S. D., Uzelac M., Mulvey R. E., Chem. Rev. 2019, 119, 8332–8405; For an overview in Non-coordinating solvents see: - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

