Mural Wnt/β-catenin signaling regulates Lama2 expression to promote neurovascular unit maturation
- PMID: 36098369
- PMCID: PMC9578690
- DOI: 10.1242/dev.200610
Mural Wnt/β-catenin signaling regulates Lama2 expression to promote neurovascular unit maturation
Abstract
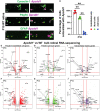
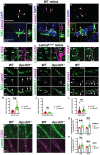
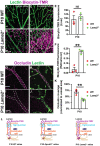
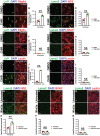
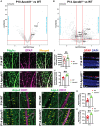
Neurovascular unit and barrier maturation rely on vascular basement membrane (vBM) composition. Laminins, a major vBM component, are crucial for these processes, yet the signaling pathway(s) that regulate their expression remain unknown. Here, we show that mural cells have active Wnt/β-catenin signaling during central nervous system development in mice. Bulk RNA sequencing and validation using postnatal day 10 and 14 wild-type versus adenomatosis polyposis coli downregulated 1 (Apcdd1-/-) mouse retinas revealed that Lama2 mRNA and protein levels are increased in mutant vasculature with higher Wnt/β-catenin signaling. Mural cells are the main source of Lama2, and Wnt/β-catenin activation induces Lama2 expression in mural cells in vitro. Markers of mature astrocytes, including aquaporin 4 (a water channel in astrocyte endfeet) and integrin-α6 (a laminin receptor), are upregulated in Apcdd1-/- retinas with higher Lama2 vBM deposition. Thus, the Wnt/β-catenin pathway regulates Lama2 expression in mural cells to promote neurovascular unit and barrier maturation.
Keywords: Blood–retinal barrier; Canonical Wnt signaling; Laminin; Mural cell; Retina; Vascular basement membrane.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Chen, J., Stahl, A., Krah, N. M., Seaward, M. R., Joyal, J.-S., Juan, A. M., Hatton, C. J., Aderman, C. M., Dennison, R. J., Willett, K. L.et al. (2012). Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS One 7, e30203. 10.1371/journal.pone.0030203 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

