Effectiveness of rapid SARS-CoV-2 genome sequencing in supporting infection control for hospital-onset COVID-19 infection: Multicentre, prospective study
- PMID: 36098502
- PMCID: PMC9596156
- DOI: 10.7554/eLife.78427
Effectiveness of rapid SARS-CoV-2 genome sequencing in supporting infection control for hospital-onset COVID-19 infection: Multicentre, prospective study
Abstract
Background: Viral sequencing of SARS-CoV-2 has been used for outbreak investigation, but there is limited evidence supporting routine use for infection prevention and control (IPC) within hospital settings.
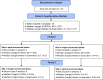
Methods: We conducted a prospective non-randomised trial of sequencing at 14 acute UK hospital trusts. Sites each had a 4-week baseline data collection period, followed by intervention periods comprising 8 weeks of 'rapid' (<48 hr) and 4 weeks of 'longer-turnaround' (5-10 days) sequencing using a sequence reporting tool (SRT). Data were collected on all hospital-onset COVID-19 infections (HOCIs; detected ≥48 hr from admission). The impact of the sequencing intervention on IPC knowledge and actions, and on the incidence of probable/definite hospital-acquired infections (HAIs), was evaluated.
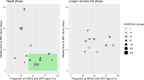
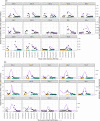
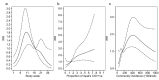
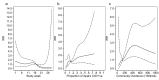
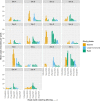
Results: A total of 2170 HOCI cases were recorded from October 2020 to April 2021, corresponding to a period of extreme strain on the health service, with sequence reports returned for 650/1320 (49.2%) during intervention phases. We did not detect a statistically significant change in weekly incidence of HAIs in longer-turnaround (incidence rate ratio 1.60, 95% CI 0.85-3.01; p=0.14) or rapid (0.85, 0.48-1.50; p=0.54) intervention phases compared to baseline phase. However, IPC practice was changed in 7.8 and 7.4% of all HOCI cases in rapid and longer-turnaround phases, respectively, and 17.2 and 11.6% of cases where the report was returned. In a 'per-protocol' sensitivity analysis, there was an impact on IPC actions in 20.7% of HOCI cases when the SRT report was returned within 5 days. Capacity to respond effectively to insights from sequencing was breached in most sites by the volume of cases and limited resources.
Conclusions: While we did not demonstrate a direct impact of sequencing on the incidence of nosocomial transmission, our results suggest that sequencing can inform IPC response to HOCIs, particularly when returned within 5 days.
Funding: COG-UK is supported by funding from the Medical Research Council (MRC) part of UK Research & Innovation (UKRI), the National Institute of Health Research (NIHR) (grant code: MC_PC_19027), and Genome Research Limited, operating as the Wellcome Sanger Institute.
Clinical trial number: NCT04405934.
Keywords: COVID-19; epidemiology; global health; healthcare-associated infection; hospital-acquired infection; human; infection control; infection prevention; infectious disease; microbiology; molecular epidemiology; viral genomics.
© 2022, Stirrup et al.
Conflict of interest statement
OS, JB, FM, AM, MP, AH, NM, TM, KS, TS, YT, NM, CP, AC, AT, RW, AD, DR, FF, SR, ML, KL, IM, BK, SH, RG, MB, AW, MB, MC, JH, GN, DP, MP, JP, CP, SR, LS, Td, ET, AC, JB No competing interests declared, GS has an unpaid role as Deputy Chair, British Medical Association London Regional Council. The author has no other competing interests to declare, MC received payment for anonymous interview conducted by Adkins Research Group. The author has no other competing interests to declare, EN holds grants by NIHR, EPSRC, MRC-UKRI , H2020, ViiV Healthcare, Pfizer and Amfar, and has received grants to attend meetings from H2020 and ViiV Healthcare, DS holds the following grants that are not specifically for the present work: COG-UK, PHE test and trace funded the sequencing aspect. HOCI funded a technician to support sequencing during study period. The author has no other competing interests to declare, FC received consulting fees from Next Gen Diagnostics LLC (during 2018/2019), received payment or honoria for lectures from University of Cambridge and Wellcome Genome Campus Advanced Courses, and received support for attending meeting and/or travel to meetings from European Congress of Clinical Microbiology &amp; Infectious Diseases (ECCMID), The American Society for Microbiology (ASM), Microbiology Society, European Congress of Clinical Microbiology &amp; Infectious Diseases (ECCMID), and the British Infection Association (BIA). The author has no other competing interests to declare, SP received consultancy fees from Pfizer (Coronavirus External Advisory Board) and Melinta Therapeutics, received payment from SVB Leerink for a round table meeting and for Mary Strauss Distinguished Public Lecture from the Fralin Biomedical Research Institute, US, and support for attending ICPIC conference, Geneva and World Health Summit, Berlin in 2021, and hold stocks or stock options in Specific Technologies (European Union Scientific Advisory Board) and Next Gen Diagnostics (Scientific Advisory Board). SP also serves as Chair, Medical Advisory Committee, Sir Jules Thorn Charitable Trust, Board member of the Wellcome SEDRIC (Surveillance and Epidemiology of Drug Resistant Consortium), and Non-Executive Director of Cambridge University Hospitals NHS Foundation Trust. The author has no other competing interests to declare, PF is a member of the SAGE hospital onset covid working group 2020-2022. The author has no other competing interests to declare
Figures



References
-
- Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, Harbarth S. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrobial Resistance and Infection Control. 2021;10:7. doi: 10.1186/s13756-020-00875-7. - DOI - PMC - PubMed
-
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. The New England Journal of Medicine. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. - DOI - PMC - PubMed
-
- Blackstone J, Stirrup O, Mapp F, Panca M, Copas A, Flowers P, Hockey L, Price J, Partridge D, Peters C, de Silva T, Nebbia G, Snell LB, McComish R, Breuer J, COVID-19 Genomics UK Consortium Protocol for the COG-UK hospital-onset COVID-19 infection (HOCI) multicentre interventional clinical study: evaluating the efficacy of rapid genome sequencing of SARS-cov-2 in limiting the spread of COVID-19 in UK NHS hospitals. BMJ Open. 2022;12:e052514. doi: 10.1136/bmjopen-2021-052514. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

