A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part
- PMID: 36098519
- PMCID: PMC9578433
- DOI: 10.1128/aac.00697-22
A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part
Abstract
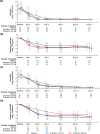
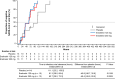
This multicenter, double-blind, phase 2a part of a phase 2/3 study assessed the efficacy and safety of ensitrelvir, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 3C-like protease inhibitor, in Japanese patients with mild-to-moderate coronavirus disease 2019 (COVID-19) or asymptomatic SARS-CoV-2 infection. Sixty-nine patients were randomized (1:1:1) to orally receive 5-day ensitrelvir fumaric acid (375 mg on day 1 followed by 125 mg daily, or 750 mg on day 1 followed by 250 mg daily) or placebo and followed up until day 28. The primary outcome was the change from baseline in the SARS-CoV-2 viral titer. A total of 16, 14, and 17 patients in the ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo groups, respectively, were included in the intention-to-treat population (mean age: 38.0 to 40.4 years). On day 4, the change from baseline in SARS-CoV-2 viral titer (log10 50% tissue culture infectious dose/mL) in patients with positive viral titer and viral RNA at baseline was greater with ensitrelvir 125 mg (mean [standard deviation], -2.42 [1.42]; P = 0.0712) and 250 mg (-2.81 [1.21]; P = 0.0083) versus placebo (-1.54 [0.74]); ensitrelvir treatment reduced SARS-CoV-2 RNA by -1.4 to -1.5 log10 copies/mL versus placebo. The viral titer and viral RNA were similar across groups on and after day 6. The median time to infectious viral clearance decreased by approximately 50 h with ensitrelvir treatment. All adverse events were mild to moderate. Ensitrelvir treatment demonstrated rapid SARS-CoV-2 clearance and was well tolerated (Japan Registry of Clinical Trials identifier: jRCT2031210350).
Keywords: COVID-19; Japanese; S-217622; SARS-CoV-2 3C-like protease inhibitor; ensitrelvir; proof-of-concept study; viral titer.
Conflict of interest statement
The authors declare a conflict of interest. H. Mukae has received funding relevant to the submitted work from Shionogi and grants from Taisho Pharma; lecture fees from Pfizer, MSD, Shionogi, and Taisho Pharma; and advisory fees from Pfizer, MSD, and Shionogi outside the submitted work. H. Yotsuyanagi has received consulting fees from Shionogi, lecture fees from Shionogi and ViiV Healthcare, and travel support from Shionogi outside the submitted work. He serves as an advisory board member of Shionogi and President of the Japanese Society of Infectious Diseases. Y. Doi has received grants from Shionogi and Entasis; consulting fees from Shionogi, Meiji Seika Pharma, Gilead Sciences, GSK, MSD, Chugai, and bioMerieux; and lecture fees from MSD, AstraZeneca, Shionogi, and Teijin Healthcare outside the submitted work and serves as an advisory board member of FujiFilm. T. Imamura, T. Sonoyama, T. Fukuhara, G. Ichihashi, T. Sanaki, K. Baba, Y. Takeda, Y. Tsuge, and T. Uehara are full-time employees of Shionogi & Co., Ltd., and may have stocks or stock options. N. Ohmagari declares no conflict of interest.
Figures


References
-
- Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, Checchi F, Perel P, Joseph S, Gibbs HP, Banerjee A, Eggo RM, Nightingale ES, O'Reilly K, Jombart T, Edmunds WJ, Rosello A, Sun FY, Atkins KE, Bosse NI, Clifford S, Russell TW, Deol AK, Liu Y, Procter SR, Leclerc QJ, Medley G, Knight G, Munday JD, Kucharski AJ, Pearson CAB, Klepac P, Prem K, Houben RMGJ, Endo A, Flasche S, Davies NG, Diamond C, van Zandvoort K, Funk S, Auzenbergs M, Rees EM, Tully DC, Emery JC, Quilty BJ, Abbott S, et al. 2020. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 8:e1003–e1017. 10.1016/S2214-109X(20)30264-3. - DOI - PMC - PubMed
-
- van Kampen JJA, van de Vijver DAMC, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, van den Akker JPC, Endeman H, Gommers D, Cornelissen JJ, Hoek RAS, van der Eerden MM, Hesselink DA, Metselaar HJ, Verbon A, de Steenwinkel JEM, Aron GI, van Gorp ECM, van Boheemen S, Voermans JC, Boucher CAB, Molenkamp R, Koopmans MPG, Geurtsvankessel C, van der Eijk AA. 2021. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 12:267. 10.1038/s41467-020-20568-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

