Pulse-Echo Quantitative US Biomarkers for Liver Steatosis: Toward Technical Standardization
- PMID: 36098640
- PMCID: PMC9613608
- DOI: 10.1148/radiol.212808
Pulse-Echo Quantitative US Biomarkers for Liver Steatosis: Toward Technical Standardization
Abstract
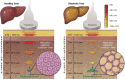
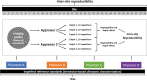
Excessive liver fat (steatosis) is now the most common cause of chronic liver disease worldwide and is an independent risk factor for cirrhosis and associated complications. Accurate and clinically useful diagnosis, risk stratification, prognostication, and therapy monitoring require accurate and reliable biomarker measurement at acceptable cost. This article describes a joint effort by the American Institute of Ultrasound in Medicine (AIUM) and the RSNA Quantitative Imaging Biomarkers Alliance (QIBA) to develop standards for clinical and technical validation of quantitative biomarkers for liver steatosis. The AIUM Liver Fat Quantification Task Force provides clinical guidance, while the RSNA QIBA Pulse-Echo Quantitative Ultrasound Biomarker Committee develops methods to measure biomarkers and reduce biomarker variability. In this article, the authors present the clinical need for quantitative imaging biomarkers of liver steatosis, review the current state of various imaging modalities, and describe the technical state of the art for three key liver steatosis pulse-echo quantitative US biomarkers: attenuation coefficient, backscatter coefficient, and speed of sound. Lastly, a perspective on current challenges and recommendations for clinical translation for each biomarker is offered.
© RSNA, 2022.
Conflict of interest statement
Figures




References
-
- Younossi ZM , Koenig AB , Abdelatif D , Fazel Y , Henry L , Wymer M . Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes . Hepatology 2016. ; 64 ( 1 ): 73 – 84 . - PubMed
-
- Eslam M , Sanyal AJ , George J ; International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease . Gastroenterology 2020. ; 158 ( 7 ): 1999 – 2014.e1 . - PubMed
-
- Chalasani N , Younossi Z , Lavine JE , et al . The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases . Hepatology 2018. ; 67 ( 1 ): 328 – 357 . - PubMed
-
- Hamer OW , Aguirre DA , Casola G , Lavine JE , Woenckhaus M , Sirlin CB . Fatty liver: imaging patterns and pitfalls . RadioGraphics 2006. ; 26 ( 6 ): 1637 – 1653 . - PubMed
-
- Brunt EM , Janney CG , Di Bisceglie AM , Neuschwander-Tetri BA , Bacon BR . Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions . Am J Gastroenterol 1999. ; 94 ( 9 ): 2467 – 2474 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

