Efficacy and Safety of Daridorexant in Older and Younger Adults with Insomnia Disorder: A Secondary Analysis of a Randomised Placebo-Controlled Trial
- PMID: 36098936
- PMCID: PMC9553778
- DOI: 10.1007/s40266-022-00977-4
Efficacy and Safety of Daridorexant in Older and Younger Adults with Insomnia Disorder: A Secondary Analysis of a Randomised Placebo-Controlled Trial
Abstract
Background and objective: The dual orexin receptor antagonist daridorexant, studied in two phase III trials, dose-dependently improved objective and subjective sleep variables and daytime functioning in adults with insomnia. Because treatment of insomnia in older adults is challenging and has limited options, the purpose of the current analysis was to further analyse the phase III trial studying the higher doses of daridorexant, those that showed efficacy (daridorexant 50 mg, daridorexant 25 mg and placebo, nightly for 3 months), and compare the safety and efficacy of daridorexant in patients aged ≥ 65 ('older adults') to those aged < 65 years ('younger adults').
Methods: Analyses by age (≥ 65 years, n = 364; < 65 years, n = 566) were performed on data from the randomised, double-blind, placebo-controlled Trial 1 in adult patients with insomnia (NCT03545191). Efficacy endpoints included a change from baseline at month 1 and month 3 in polysomnography-measured wake after sleep onset (WASO) and latency to persistent sleep (LPS), self-reported total sleep time (sTST) and daytime functioning assessed using the validated Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ). Safety endpoints included adverse events and the Visual Analog Scale for morning sleepiness.
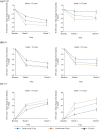
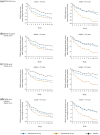
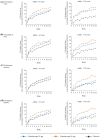
Results: At baseline, mean [standard deviation] WASO was numerically greater (110 [39] vs 92 [38] min) in older than younger adults, while LPS was comparable (~ 65 min). Mean baseline IDSIQ total and all domain scores were numerically lower (i.e. better) in older adults. Daridorexant caused similar reductions in WASO and LPS, and similar increases in sTST, from baseline, in both age groups; improvements were numerically greater with daridorexant 50 mg than 25 mg. At month 3, daridorexant 50 mg, compared with placebo, decreased WASO by a least-squares mean of 19.6 (95% confidence interval 9.7, 29.5) in older patients versus 17.4 min (10.7, 24.0) in younger patients and decreased LPS by a least-squares mean of 14.9 (7.5, 22.3) in older patients versus 9.7 min (3.7, 15.7) in younger patients. Daridorexant 50 mg increased sTST from baseline to month 3 by a least-squares mean of 59.9 (49.6, 70.3) in older patients versus 57.1 min (48.9, 65.3) in younger patients. Daridorexant 50 mg progressively improved IDSIQ total and domain scores from week 1 onwards similarly in both groups; daridorexant 25 mg improved IDSIQ scores, but only in younger adults. In both age groups, in comparison with placebo, the overall incidence of adverse events was comparable, and there were fewer falls on daridorexant. Daridorexant improved Visual Analog Scale morning sleepiness in both groups; daridorexant 50 mg increased the mean (standard deviation) Visual Analog Scale morning sleepiness score by 15.9 (20.7) in older adults and by 14.9 (18.7) in younger adults from baseline to month 3. In older adults, there was one case of sleep paralysis, and no cases of narcolepsy, cataplexy, or complex sleep behaviour.
Conclusions: In older patients with insomnia, as in younger patients, the efficacy of daridorexant is maximal on night-time and daytime variables at the higher dose of 50 mg. Older patients particularly require this dose to improve daytime functioning. Older patients are not at an increased risk of adverse events or residual effects the next morning after night-time administration of daridorexant, even at 50 mg. The dose of daridorexant does not need to be decreased for older patients.
Clinical trial registration: ClinicalTrials.gov (NCT03545191) [first posted: 4 June, 4 2018], https://clinicaltrials.gov/ct2/show/NCT03545191 .
Plain language summary
The burden of chronic insomnia (difficulty in falling/staying asleep or not getting enough sleep) increases with age yet treatment options in older patients are limited. In older patients, because of a risk of side effects, guidelines suggest caution when prescribing sleep medications and, for some drugs, recommend starting at a lower dose. Daridorexant was approved in 2022 for the treatment of insomnia in adults following positive results in two trials that showed daridorexant significantly improved night-time sleep and daytime functioning over 3 months of treatment in adults with insomnia. Approximately 40% of patients taking part in these trials were aged 65 years or older. This current analysis compared the safety and benefits of daridorexant in older adults (aged at least 65 years) and younger adults (aged less than 65 years) in the trial that administered the highest two doses of daridorexant, 25 and 50 mg. The results showed that the benefits of daridorexant were comparable in both age groups over 3 months; compared with placebo, daridorexant improved night-time sleep (reduced time awake during the night, reduced time to fall asleep and increased total sleep time) and daytime functioning—patients had less daytime sleepiness and a better mood and feeling of alertness. In older patients, the benefits, particularly for daytime functioning, were greatest at the higher 50-mg dose, without any increase in side effects. Both doses of daridorexant were equally well tolerated in the two age groups, indicating that treatment with daridorexant at 50 mg can be safely started in older patients.
© 2022. The Author(s).
Conflict of interest statement
IF reports consulting fees from Bayer, Bioproject, Hennig, Idorsia Pharmaceuticals, Jazz Pharmaceuticals, and STADA and speaker activity/reimbursement from Hennig, Idorsia Pharmaceuticals and Medice. CLAB reports consultancy fees from Bioproject, Takeda and Jazz Pharmaceuticals during the conduct of the trials. DM has been a clinical investigator for Apnimed, Eisai, Idorsia Pharmaceuticals, Imbrium, Janssen, Jazz Pharmaceuticals, Merck, Sage, Takeda and Vanda. WVM reports scientific advisor fees from Idorsia Pharmaceuticals and Janssen, and royalties from Wolter Kluwer. DSK and SP are employees of Idorsia Pharmaceuticals. IDSIQ was developed by Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A Hall M, Moul DE, Nofzinger EA and Kupfer DJ of the University of Pittsburgh and as amended by Idorsia Pharmaceuticals Ltd. IDSIQ© 2020, University of Pittsburg. All rights reserved. IDSIQ-14 derivative created 2020 by Idorsia Pharmaceuticals Ltd under license and distributed by Idorsia Pharmaceuticals Ltd under license.
Figures

References
-
- United Nations Department of Economics and Social Affairs Population Division. World population ageing 2019. Available from: https://www.un.org/en/development/desa/population/publications/pdf/agein.... Accessed 30 Aug 2022.
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Publishing; 2013.
-
- Ito E, Inoue Y. The international classification of sleep disorders, third edition. Am Acad Sleep Med. 2015;73(6):916–923. - PubMed

