Co-ordinated control of the ADP-heptose/ALPK1 signalling network by the E3 ligases TRAF6, TRAF2/c-IAP1 and LUBAC
- PMID: 36098982
- PMCID: PMC9704527
- DOI: 10.1042/BCJ20220401
Co-ordinated control of the ADP-heptose/ALPK1 signalling network by the E3 ligases TRAF6, TRAF2/c-IAP1 and LUBAC
Abstract
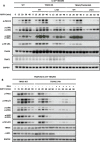
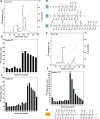
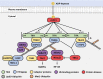
ADP-heptose activates the protein kinase ALPK1 triggering TIFA phosphorylation at Thr9, the recruitment of TRAF6 and the subsequent production of inflammatory mediators. Here, we demonstrate that ADP-heptose also stimulates the formation of Lys63- and Met1-linked ubiquitin chains to activate the TAK1 and canonical IKK complexes, respectively. We further show that the E3 ligases TRAF6 and c-IAP1 operate redundantly to generate the Lys63-linked ubiquitin chains required for pathway activation, which we demonstrate are attached to TRAF6, TRAF2 and c-IAP1, and that c-IAP1 is recruited to TIFA by TRAF2. ADP-heptose also induces activation of the kinase TBK1 by a TAK1-independent mechanism, which require TRAF2 and TRAF6. We establish that ALPK1 phosphorylates TIFA directly at Thr177 as well as Thr9 in vitro. Thr177 is located within the TRAF6-binding motif and its mutation to Asp prevents TRAF6 but not TRAF2 binding, indicating a role in restricting ADP-heptose signalling. We conclude that ADP-heptose signalling is controlled by the combined actions of TRAF2/c-IAP1 and TRAF6.
Keywords: ADP-heptose; ALPK1; TAK1; TBK1; TIFA; TRAF.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures






References
-
- Stein, S.C., Faber, E., Bats, S.H., Murillo, T., Speidel, Y., Coombs, N.et al. (2017) Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog. 13, e1006514 10.1371/journal.ppat.1006514 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

