Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation
- PMID: 36099053
- PMCID: PMC9675439
- DOI: 10.1172/jci.insight.148309
Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation
Retraction in
-
Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation.JCI Insight. 2024 Jun 10;9(11):e181492. doi: 10.1172/jci.insight.181492. JCI Insight. 2024. PMID: 38855870 Free PMC article. No abstract available.
Abstract
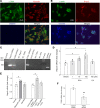
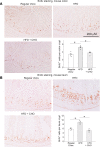
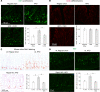
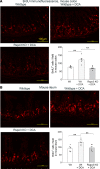
A high-fat diet (HFD) contributes to the increased incidence of colorectal cancer, but the mechanisms are unclear. We found that R-spondin 3 (Rspo3), a ligand for leucine-rich, repeat-containing GPCR 4 and 5 (LGR4 and LGR5), was the main subtype of R-spondins and was produced by myofibroblasts beneath the crypts in the intestine. HFD upregulated colonic Rspo3, LGR4, LGR5, and β-catenin gene expression in specific pathogen-free rodents, but not in germ-free mice, and the upregulations were prevented by the bile acid (BA) binder cholestyramine or antibiotic treatment, indicating mediation by both BA and gut microbiota. Cholestyramine or antibiotic treatments prevented HFD-induced enrichment of members of the Lachnospiraceae and Rumincoccaceae, which can transform primary BA into secondary BA. Oral administration of deoxycholic acid (DCA), or inoculation of a combination of the BA deconjugator Lactobacillus plantarum and 7α-dehydroxylase-containing Clostridium scindens with an HFD to germ-free mice increased serum DCA and colonic Rspo3 mRNA levels, indicating that formation of secondary BA by gut microbiota is responsible for HFD-induced upregulation of Rspo3. In primary myofibroblasts, DCA increased Rspo3 mRNA via TGR5. Finally, we showed that cholestyramine or conditional deletion of Rspo3 prevented HFD- or DCA-induced intestinal proliferation. We conclude that secondary BA is responsible for HFD-induced upregulation of Rspo3, which, in turn, mediates HFD-induced intestinal epithelial proliferation.
Keywords: Colorectal cancer; Gastroenterology; Mouse stem cells.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

