Epipharyngeal Abrasive Therapy Down-regulates the Expression of Cav1.2: A Key Molecule in Influenza Virus Entry
- PMID: 36099101
- PMCID: PMC9463883
- DOI: 10.21873/invivo.12967
Epipharyngeal Abrasive Therapy Down-regulates the Expression of Cav1.2: A Key Molecule in Influenza Virus Entry
Abstract
Background/aim: Influenza A virus (IAV) infection causes an inflammatory response to the respiratory mucosa. The viral glycoprotein hemagglutinin (HA) binds to the sialylated voltage-dependent Ca2+ channel (Cav1.2) in ciliated epithelium. The binding of HA and sialylated Cav1.2 is considered essential to IAV infection, entry, and IAV-induced Ca2+ oscillation. The epipharynx comprises the ciliated epithelium, which is the initial target for viruses that cause upper respiratory tract infections. Previously, we showed that epipharyngeal abrasive therapy (EAT), a treatment for chronic epipharyngitis in Japan, which scratches the epipharyngeal mucosa with a cotton swab containing zinc chloride, induces squamous metaplasia. In this study, we evaluated whether squamous metaplasia by EAT affects the expression patterns of Cav1.2.
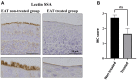
Patients and methods: The study subjects were seven patients who had not been treated with EAT and 11 patients who had. For the immunohistochemical assessment of the epipharyngeal mucosa, the staining intensity of Cav1.2 was described using the immunohistochemical score (IHC score).
Results: The IHC scores for Cav1.2 in the EAT-treated group was 4.19-fold lower than those in the non-treated group (p=0.0034).
Conclusion: EAT down-regulates the expression of Cav1.2, a key cell surface molecule in influenza virus entry via squamous metaplasia. Thus, EAT may be a simple method for preventing influenza infection.
Keywords: Epipharyngeal abrasive therapy (EAT); chronic epipharyngitis; influenza; influenza A virus (IAV); voltage-dependent Ca2+ channel (Cav1.2).
Copyright © 2022, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare that they have no competing interests in relation to this study.
Figures


References
-
- Fujioka Y, Nishide S, Ose T, Suzuki T, Kato I, Fukuhara H, Fujioka M, Horiuchi K, Satoh AO, Nepal P, Kashiwagi S, Wang J, Horiguchi M, Sato Y, Paudel S, Nanbo A, Miyazaki T, Hasegawa H, Maenaka K, Ohba Y. A sialylated voltage-dependent Ca2+ channel binds hemagglutinin and mediates influenza A virus entry into mammalian cells. Cell Host Microbe. 2018;23(6):809–818.e5. doi: 10.1016/j.chom.2018.04.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
