The Antiseptic and Antineoplastic Agent Taurolidine Modulates Key Leukocyte Functions
- PMID: 36099103
- PMCID: PMC9463896
- DOI: 10.21873/invivo.12933
The Antiseptic and Antineoplastic Agent Taurolidine Modulates Key Leukocyte Functions
Abstract
Background/aim: Although taurolidine is known to exert a wide spectrum of biological actions, its effects on immune cells have not been characterized in detail. In this study, we investigated the ex vivo effects of taurolidine on relevant innate and adaptive immune cell functions.
Materials and methods: Leukocyte functions in whole blood were assessed following treatment with various taurolidine concentrations. Viability of peripheral blood mononuclear cells (PBMCs) and granulocytes was measured using the WST-1 assay. PBMC function was assessed by measuring TNFα and IFNγ production after stimulation with lipopolysaccharide (LPS) or Candida, respectively. Reactive oxygen species (ROS) production by granulocytes was measured in whole blood using luminol-enhanced chemiluminescence. Granulocyte degranulation and activation were evaluated by membrane expression of degranulation (CD63, CD66B) and adhesion markers (CD62L, CD11b) using immunofluorescent staining followed by flow-cytometric analysis.
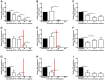
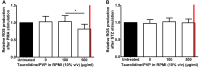
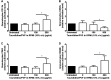
Results: Taurolidine decreased viability of PBMCs and granulocytes: after 2 h, IC50 concentrations were 500 and 520 μg/ml, respectively. Following prolonged exposure (≥24 h) of PBMCs, the IC50 concentrations declined to 40 μg/ml. PBMC cytokine production significantly decreased at taurolidine concentrations below the cytotoxic threshold, whereas no changes in ROS production were observed. The expression of all granulocyte adhesion and degranulation markers increased at concentrations higher than 500 μg/ml (the cytotoxic level of taurolidine).
Conclusion: Taurolidine exhibits a dose- and time-dependent cytotoxicity toward PBMCs and granulocytes. The effects on PBMCs, as exemplified by a decrease in cytokine production, occurred below the toxic threshold, whereas granulocyte function (ROS production) remained unchanged at these taurolidine concentrations. Granulocyte activation and degranulation markers only increased at cytotoxic taurolidine concentrations.
Keywords: CD11b; CD62L; CD63; CD66B; PBMC; Taurolidine; cell markers; cytokines; cytotoxicity; granulocyte; leukocytes; neutrophil; reactive oxygen species.
Copyright © 2022, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
GW reports grants from Geistlich Pharma AG, Fresenius Kabi, Baxter international and BBraun Medical outside the submitted work. GW is a consultant for Shire. YW reports grants from Geistlich Pharma AG outside the submitted work.
Figures

Similar articles
-
Taurolidine and povidone-iodine induce different types of cell death in malignant pleural mesothelioma.Lung Cancer. 2007 Jun;56(3):327-36. doi: 10.1016/j.lungcan.2007.01.024. Epub 2007 Mar 23. Lung Cancer. 2007. PMID: 17383050
-
Induction of reactive oxygen intermediates-dependent programmed cell death in human malignant ex vivo glioma cells and inhibition of the vascular endothelial growth factor production by taurolidine.J Neurosurg. 2005 Jun;102(6):1055-68. doi: 10.3171/jns.2005.102.6.1055. J Neurosurg. 2005. PMID: 16028765
-
Enhancement of Fas-ligand-mediated programmed cell death by taurolidine.Anticancer Res. 2003 May-Jun;23(3B):2309-14. Anticancer Res. 2003. PMID: 12894508
-
Redox-directed cancer therapeutics: Taurolidine and Piperlongumine as broadly effective antineoplastic agents (review).Int J Oncol. 2014 Oct;45(4):1329-36. doi: 10.3892/ijo.2014.2566. Epub 2014 Jul 28. Int J Oncol. 2014. PMID: 25175943 Free PMC article. Review.
-
The evolving role of taurolidine in cancer therapy.Ann Surg Oncol. 2010 Apr;17(4):1135-43. doi: 10.1245/s10434-009-0867-9. Epub 2009 Dec 29. Ann Surg Oncol. 2010. PMID: 20039217 Review.
Cited by
-
Exploring Water-Soluble South African Tulbaghia violacea Harv Extract as a Therapeutic Approach for Triple-Negative Breast Cancer Metastasis.Curr Issues Mol Biol. 2024 Sep 26;46(10):10806-10828. doi: 10.3390/cimb46100642. Curr Issues Mol Biol. 2024. PMID: 39451522 Free PMC article.
References
-
- Bühler HU, Mikic St, Wicki O. [A new surgical lavage] Helv Chir Acta. 1978;45(1-2):143–145. - PubMed
-
- Linder MM, Ott W, Wesch G. [Antibacterial therapy of purulent peritonitis: a prospective randomized study on the effects of antibiotics and taurolin, a new chemotherapeutic and antiendotoxic agent (author’s transl)] Chir Forum Exp Klin. 1980;Forsch:67–71. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
