A Randomized, Placebo-Controlled Study of Methotrexate to Increase Response Rates in Patients with Uncontrolled Gout Receiving Pegloticase: Primary Efficacy and Safety Findings
- PMID: 36099211
- PMCID: PMC10107774
- DOI: 10.1002/art.42335
A Randomized, Placebo-Controlled Study of Methotrexate to Increase Response Rates in Patients with Uncontrolled Gout Receiving Pegloticase: Primary Efficacy and Safety Findings
Abstract
Objective: To assess efficacy, safety, pharmacokinetics, and immunogenicity of pegloticase plus methotrexate (MTX) versus pegloticase plus placebo cotreatment for uncontrolled gout in a randomized, placebo-controlled, double-blind trial.
Methods: This study included adults with uncontrolled gout, defined as serum urate ≥7 mg/dl, oral urate-lowering therapy failure or intolerance, and presence of ongoing gout symptoms including ≥1 tophus, ≥2 flares in the past 12 months, or gouty arthritis. Key exclusion criteria included MTX contraindication, current immunosuppressant use, G6PDH deficiency, and estimated glomerular filtration rate <40 ml/minute/1.73 m2 . Patients were randomized 2:1 to 52 weeks of pegloticase (8 mg biweekly) with either oral MTX (15 mg/week) or placebo. The primary end point was the proportion of treatment responders during month 6 (defined as serum urate <6 mg/dl for ≥80% of visits during weeks 20-24). Efficacy was evaluated in all randomized patients (intent-to-treat population), and safety was evaluated in all patients receiving ≥1 blinded MTX or placebo dose.
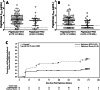
Results: A total of 152 patients were randomized, 100 to receive pegloticase plus MTX, 52 to receive pegloticase plus placebo. Significantly higher treatment response occurred during month 6 in the MTX group versus the placebo group (71.0% [71 of 100 patients] versus 38.5% [20 of 52 patients], respectively; between-group difference 32.3% [95% confidence interval 16.3%, 48.3%]) (P < 0.0001 for between-group difference). During the first 6 months of pegloticase plus MTX or pegloticase plus placebo treatment, 78 (81.3%) of 96 MTX patients versus 47 (95.9%) of 49 placebo patients experienced ≥1 adverse event (AE), most commonly gout flare (64 [66.7%] of 96 MTX patients and 34 [69.4%] of 49 placebo patients). Reports of AEs and serious AEs were comparable between groups, but the infusion reaction rate was considerably lower with MTX cotherapy (4.2% [4 of 96 MTX patients, including 1 patient who had anaphylaxis]) than with placebo cotherapy (30.6% [15 of 49 placebo patients, 0 who had anaphylaxis]) (P < 0.001). Antidrug antibody positivity was also lower in the MTX group.
Conclusion: MTX cotherapy markedly increased pegloticase response rate over placebo (71.0% versus 38.5%) during month 6 with no new safety signals. These findings verify higher treatment response rate, lower infusion reaction incidence, and lower immunogenicity when pegloticase is coadministered with MTX.
Trial registration: ClinicalTrials.gov NCT03994731.
© 2022 Horizon Therapeutics PLC. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures



References
-
- Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306:711–20. - PubMed
-
- Keenan RT, Botson JK, Masri KR, et al. The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review. Semin Arthritis Rheum 2021;51:347–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

