In the Body's Eye: The computational anatomy of interoceptive inference
- PMID: 36099315
- PMCID: PMC9506608
- DOI: 10.1371/journal.pcbi.1010490
In the Body's Eye: The computational anatomy of interoceptive inference
Abstract
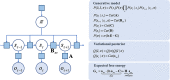
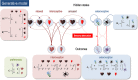
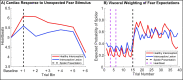
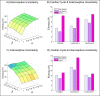
A growing body of evidence highlights the intricate linkage of exteroceptive perception to the rhythmic activity of the visceral body. In parallel, interoceptive inference theories of affective perception and self-consciousness are on the rise in cognitive science. However, thus far no formal theory has emerged to integrate these twin domains; instead, most extant work is conceptual in nature. Here, we introduce a formal model of cardiac active inference, which explains how ascending cardiac signals entrain exteroceptive sensory perception and uncertainty. Through simulated psychophysics, we reproduce the defensive startle reflex and commonly reported effects linking the cardiac cycle to affective behaviour. We further show that simulated 'interoceptive lesions' blunt affective expectations, induce psychosomatic hallucinations, and exacerbate biases in perceptual uncertainty. Through synthetic heart-rate variability analyses, we illustrate how the balance of arousal-priors and visceral prediction errors produces idiosyncratic patterns of physiological reactivity. Our model thus offers a roadmap for computationally phenotyping disordered brain-body interaction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

