A genetically modified minipig model for Alzheimer's disease with SORL1 haploinsufficiency
- PMID: 36099918
- PMCID: PMC9512670
- DOI: 10.1016/j.xcrm.2022.100740
A genetically modified minipig model for Alzheimer's disease with SORL1 haploinsufficiency
Abstract
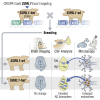
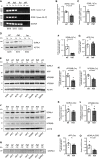
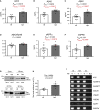
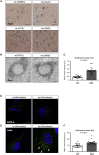
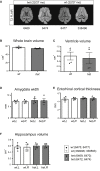
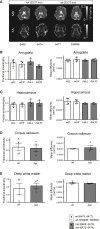
The established causal genes in Alzheimer's disease (AD), APP, PSEN1, and PSEN2, are functionally characterized using biomarkers, capturing an in vivo profile reflecting the disease's initial preclinical phase. Mutations in SORL1, encoding the endosome recycling receptor SORLA, are found in 2%-3% of individuals with early-onset AD, and SORL1 haploinsufficiency appears to be causal for AD. To test whether SORL1 can function as an AD causal gene, we use CRISPR-Cas9-based gene editing to develop a model of SORL1 haploinsufficiency in Göttingen minipigs, taking advantage of porcine models for biomarker investigations. SORL1 haploinsufficiency in young adult minipigs is found to phenocopy the preclinical in vivo profile of AD observed with APP, PSEN1, and PSEN2, resulting in elevated levels of β-amyloid (Aβ) and tau preceding amyloid plaque formation and neurodegeneration, as observed in humans. Our study provides functional support for the theory that SORL1 haploinsufficiency leads to endosome cytopathology with biofluid hallmarks of autosomal dominant AD.
Keywords: Alzheimer’s disease; CRISPR-Cas9; SORL1; SORLA; genome editing; large animal model; retromer-dependent endosomal recycling.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.B. and M.D. are employees of AbbVie and own AbbVie stock. AbbVie participated in the design and study conduct for this research as well as in the interpretation of data, review, and approval of the publication. Ellegaard Göttingen Minipigs A/S has the commercialization rights to the genetically altered Göttingen minipig SORL1 model. S.A.S. is a co-founder of Retromer Therapeutics, has equity in the company, and is a paid consultant to the company. O.M.A. also has commercial interests in Retromer Therapeutics, but the company was not involved in any aspects of the study.
Figures


References
-
- Jensen M., Schröder J., Blomberg M., Engvall B., Pantel J., Ida N., Basun H., Wahlund L.O., Werle E., Jauss M., et al. Cerebrospinal fluid A beta42 is increased early in sporadic Alzheimer's disease and declines with disease progression. Ann. Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. - DOI - PubMed
-
- Maia L.F., Kaeser S.A., Reichwald J., Lambert M., Obermüller U., Schelle J., Odenthal J., Martus P., Staufenbiel M., Jucker M. Increased CSF Abeta during the very early phase of cerebral Abeta deposition in mouse models. EMBO Mol. Med. 2015;7:895–903. doi: 10.15252/emmm.201505026. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

