CRB1-Associated Retinal Dystrophies: Genetics, Clinical Characteristics, and Natural History
- PMID: 36099972
- PMCID: PMC10555856
- DOI: 10.1016/j.ajo.2022.09.002
CRB1-Associated Retinal Dystrophies: Genetics, Clinical Characteristics, and Natural History
Abstract
Purpose: To analyze the clinical characteristics, natural history, and genetics of CRB1-associated retinal dystrophies.
Design: Multicenter international retrospective cohort study.
Methods: Review of clinical notes, ophthalmic images, and genetic testing results of 104 patients (91 probands) with disease-causing CRB1 variants. Macular optical coherence tomography (OCT) parameters, visual function, fundus characteristics, and associations between variables were the main outcome measures.
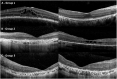
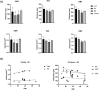
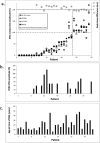
Results: The mean age of the cohort at the first visit was 19.8 ± 16.1 (median 15) years, with a mean follow-up of 9.6 ± 10 years. Based on history, imaging, and clinical examination, 26 individuals were diagnosed with retinitis pigmentosa (RP; 25%), 54 with early-onset severe retinal dystrophy / Leber congenital amaurosis (EOSRD/LCA; 52%), and 24 with macular dystrophy (MD; 23%). Severe visual impairment was most frequent after 40 years of age for patients with RP and after 20 years of age for EOSRD/LCA. Longitudinal analysis revealed a significant difference between baseline and follow-up best-corrected visual acuity in the 3 subcohorts. Macular thickness decreased in most patients with EOSRD/LCA and MD, whereas the majority of patients with RP had increased perifoveal thickness.
Conclusions: A subset of individuals with CRB1 variants present with mild, adult-onset RP. EOSRD/LCA phenotype was significantly associated with null variants, and 167_169 deletion was exclusively present in the MD cohort. The poor OCT lamination may have a degenerative component, as well as being congenital. Disease symmetry and reasonable window for intervention highlight CRB1 retinal dystrophies as a promising target for trials of novel therapeutics.
Keywords: CRB1; Early Onset Severe Retinal Dystrophy; Fundus autofluorescence; Gene therapy; Genotype; LCA; Macular dystrophy; Optical coherence tomography; Phenotype; RP; Retinal dystrophy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

