Comparative analysis of humoral responses to BNT162b2 vaccine among patients with hematologic disorders and organ transplant recipients
- PMID: 36100196
- PMCID: PMC9465495
- DOI: 10.1016/j.trim.2022.101713
Comparative analysis of humoral responses to BNT162b2 vaccine among patients with hematologic disorders and organ transplant recipients
Abstract
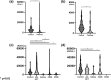
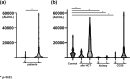
Vaccination against SARS-COV-2 is considered the most promising approach to curbing the pandemic. Patients with an immunocompromised state, such as those with hematological malignancies and organ transplantation recipients, are considered more susceptible to infection, but these at-risk patients were underrepresented in early clinical trials for vaccination. Although a growing body of studies suggests that the humoral response to COVID-19 vaccination in each of these at-risk groups of patients may be suboptimal in comparison to healthy controls, a clinical and strategic information for the further comparative analysis among these groups is not fully described. The humoral responses after two doses of BNT162b2 vaccination were evaluated in a total of 187 patients either with allogeneic hematopoietic transplantation, with renal transplantation, with anti-CD20 antibody therapy, or with anti-CD38 antibody therapy, and in 66 healthy controls. The early response at one to three months after vaccination was significantly inferior among patients with renal transplantation, patients with anti-CD20 antibody therapy, and patients with anti-CD38 antibody therapy in comparison to healthy control. But the patients with allogeneic hematopoietic transplantation showed early humoral response comparable to healthy control. The late response at 6 months after vaccination was still suboptimal among patients with renal transplantation and patients with anti-CD20 therapy. Among our patient group, renal transplant recipients had the lowest antibody titers after vaccination regardless of timing of vaccination. Patients who had received allogeneic hematopoietic transplantation attained a comparable serological response to the control group especially if they are vaccinated >300 days after transplantation, but the response was suboptimal if the vaccination was within 300 days after transplantation. Our results may provide policy makers with critical information for the further stratification of at-risk groups, helping contribute to a better allocation of resources, including additional booster vaccination.
Keywords: Anti-CD20 antibody; COVID-19; Hematopoietic transplantation; Kidney transplantation; Vaccination.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients.J Am Soc Nephrol. 2021 Sep;32(9):2153-2158. doi: 10.1681/ASN.2021040490. Epub 2021 Jun 16. J Am Soc Nephrol. 2021. PMID: 34135083 Free PMC article.
-
The humoral immune response to the BNT 162B2 vaccine in pediatrics on renal replacement therapy.Pediatr Transplant. 2024 May;28(3):e14712. doi: 10.1111/petr.14712. Pediatr Transplant. 2024. PMID: 38553800
-
Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation.Transplant Cell Ther. 2022 Apr;28(4):214.e1-214.e11. doi: 10.1016/j.jtct.2022.01.019. Epub 2022 Jan 31. Transplant Cell Ther. 2022. PMID: 35092892 Free PMC article.
-
Seroconversion rate after primary vaccination with two doses of BNT162b2 versus mRNA-1273 in solid organ transplant recipients: a systematic review and meta-analysis.Nephrol Dial Transplant. 2022 Jul 26;37(8):1566-1575. doi: 10.1093/ndt/gfac174. Nephrol Dial Transplant. 2022. PMID: 35544087 Free PMC article.
-
Update on COVID-19 vaccination in pediatric solid organ transplant recipients.Pediatr Transplant. 2022 Aug;26(5):e14235. doi: 10.1111/petr.14235. Epub 2022 Jan 20. Pediatr Transplant. 2022. PMID: 35060251 Review.
References
-
- Gavriatopoulou M., Terpos E., Kastritis E., Briasoulis A., Gumeni S., Ntanasis-Stathopoulos I., Sklirou A.D., Malandrakis P., Eleutherakis-Papaiakovou E., Migkou M., Trougakos I.P., Dimopoulos M.A. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin. Exp. Med. 2021:1–5. - PMC - PubMed
-
- Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., Valluri S.R., Pan K., Angulo F.J., Jodar L., McLaughlin J.M. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. - PMC - PubMed
-
- Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Yang Q., Liberator P., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Gruber W.C., Jansen K.U. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021;385(19):1761–1773. - PMC - PubMed
-
- W.H. Organization WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/ (Accessed November 21 2021)
-
- C.P.A. Office Novel Coronavirus Vaccines. 2021. https://japan.kantei.go.jp/ongoingtopics/vaccine.html (Accessed November 21 2021)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

